Published online Jun 21, 2006. doi: 10.3748/wjg.v12.i23.3782
Revised: February 28, 2006
Accepted: March 13, 2006
Published online: June 21, 2006
- Citation: Mathonnet M, Descottes B, Valleix D, Labrousse F, Truffinet V, Denizot Y. Quantitative analysis using ELISA of vascular endothelial growth factor and basic fibroblast growth factor in human colorectal cancer, liver metastasis of colorectal cancer and hepatocellular carcinoma. World J Gastroenterol 2006; 12(23): 3782-3783
- URL: https://www.wjgnet.com/1007-9327/full/v12/i23/3782.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i23.3782
Angiogenesis consists of the sprouting of capillaries from pre-existing vessels[1]. It is well-known that tumor growth is angiogenesis-dependent. Vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) stimulated vascular endothelial cell proliferation and are involved in the neoplastic angiogenesis of several types of tumors including those of the intestinal tract[1-5]. Authors usually investigated VEGF and bFGF protein expressions using immunohistochemistry or Western blotting and VEGF and bFGF transcripts using reverse transcriptase polymerase chain reaction (RT-PCR). We recently reported in a previous issue of the World Journal of Gastroenterology that cirrhotic liver tissue levels of these two angiogenic growth factors can be easily evaluated by an enzyme-linked immunosorbent assay (ELISA)[6]. In order to confirm if this technical approach was suitable for tumors of the intestinal tract, we performed quantitative analysis of VEGF and bFGF in surgically resected tumors of colorectal cancer, liver metastases of colorectal cancer and hepatocellular carcinomas (HCC).
The procedure of the present study followed the rules edited by the French National Ethics. A total of 86 patients were investigated in the Department of Surgery of Limoges’ CHU. Among them, 29 patients (18 men and 11 women, mean age 76 years) had a primary colorectal cancer, 30 patients (15 men and 15 women, mean age 64 years) had liver metastasis of colorectal cancer and 27 patients (26 men and 1 woman, mean age 62 years) had an HCC. Specimens for the tumor and the adjacent tissue were obtained during the surgical procedure and frozen at -80°C until used. Tissue samples were homogenized in potassium phosphate buffer and VEGF and bFGF contents were detected using specific ELISA assays (DuoSet®, R&D Systems, Europe) according to the manufacturer’s recommendations. Results in picogram per milligram (pg/mg) of wet weight are reported as mean ± SE. Differences in contents of VEGF and bFGF among tumor and the adjacent non-tumor tissue were analyzed by the Student t-test for paired data. A P value less than 0.05 was considered statistically significant.
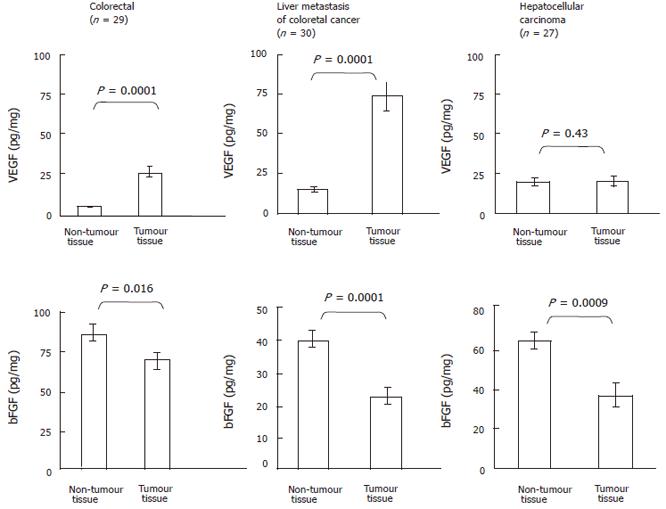
As shown in Figure 1 (upper panel), VEGF contents were significantly elevated (P = 0.0001) in colorectal tumor (43.1 ± 9.0 pg/mg, n = 29) than that in the adjacent non-tumor tissue (5.3 ± 0.8 pg/mg, n = 29). Similarly, VEGF levels were markedly elevated (P = 0.0001) in liver metastasis of colorectal cancer (72.7 ± 9.5 pg/mg, n = 30) compared to the control tissue (14.7 ± 1.5 pg/mg, n = 30). In contrast, no significant differences were observed in VEGF levels between HCC (19.0 ± 2.3 pg/mg, n = 27) and the adjacent non-tumor tissue (18.4 ± 3.4 pg/mg, n = 27) (P = 0.43). As shown in Figure 1 (lower panel), bFGF contents were significantly reduced (P = 0.016) in colorectal tumor (67.0 ± 6.0 pg/mg, n = 29) compared to the adjacent non-tumor tissue (85.0 ± 6.0 pg/mg, n = 29). Similarly, VEGF levels were obviously lower (P = 0.0001) in liver metastasis of colorectal cancer (21.4 ± 3.4 pg/mg, n = 30) than that in the control tissue (39.3 ± 3.0 pg/mg, n = 30). VEGF levels were also reduced (P = 0.0009) in HCC (35.4 ± 7.3 pg/mg, n = 27) as compared with the adjacent non-tumor tissue (63.0 ± 4.6 pg/mg, n = 27).
The present study highlights that the ELISA method is a valuable method for the quantitative analysis of bFGF and VEGF contents in tumors located in the intestinal tract. We observed that bFGF contents in colorectal tumor, liver metastasis and HCC were significantly lower compared to the adjacent non-tumor tissues. This observation confirms a previous report investigating bFGF levels in human colorectal cancer[4]. At first sight, these results might be surprising since studies reported the involvement of bFGF in tumor growth. In fact, even though bFGF lacks a leader sequence for secretion, it is suggested that bFGF (localized to secretory granules) can be secreted by an alternative secretion pathway, accumulates in the extracellular matrix (ECM), from where it can be released by ECM-degrading enzymes. Our observation might, thus, be explained by secretion events inside tumors (leading to low bFGF tumor tissue content) and bFGF accumulation in tumor periphery (leading to high bFGF non-tumor tissue content). VEGF protein levels were markedly elevated in colorectal tumor and in their liver metastasis. These results are in agreement with the well known involvement of VEGF in colorectal tumor growth[7]. In contrast, VEGF protein levels were not elevated in HCC compared with surrounding non-tumor tissue. These results contrast with a previous study[2], but might be explain by the fact that a large number of our patients developed HCC in a cirrhotic liver; VEGF levels being elevated in cirrhotic tissue as compared with HCC ones[3,6].
In conclusion, results obtained with the ELISA procedure corroborate those obtained with other methods. It is, thus, tempting to speculate that the ELISA method would also be valuable for the quantitative analysis of other angiogenic growth factors and cytokines in tumors located in the intestinal tract.
S- Editor Wang J L- Editor Kumar M E- Editor Liu Y
| 1. | Bussolino F, Albini A, Camussi G, Presta M, Viglietto G, Ziche M, Persico G. Role of soluble mediators in angiogenesis. Eur J Cancer. 1996;32A:2401-2412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 64] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 2. | Tang TC, Poon RT, Lau CP, Xie D, Fan ST. Tumor cyclooxygenase-2 levels correlate with tumor invasiveness in human hepatocellular carcinoma. World J Gastroenterol. 2005;11:1896-1902. [PubMed] |
| 3. | Deli G, Jin CH, Mu R, Yang S, Liang Y, Chen D, Makuuchi M. Immunohistochemical assessment of angiogenesis in hepatocellular carcinoma and surrounding cirrhotic liver tissues. World J Gastroenterol. 2005;11:960-963. [PubMed] |
| 4. | Landriscina M, Cassano A, Ratto C, Longo R, Ippoliti M, Palazzotti B, Crucitti F, Barone C. Quantitative analysis of basic fibroblast growth factor and vascular endothelial growth factor in human colorectal cancer. Br J Cancer. 1998;78:765-770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | El-Assal ON, Yamanoi A, Ono T, Kohno H, Nagasue N. The clinicopathological significance of heparanase and basic fibroblast growth factor expressions in hepatocellular carcinoma. Clin Cancer Res. 2001;7:1299-1305. [PubMed] |
| 6. | Mathonnet M, Descottes B, Valleix D, Labrousse F, Denizot Y. VEGF in hepatocellular carcinoma and surrounding cirrhotic liver tissues. World J Gastroenterol. 2006;12:830-831. [PubMed] |
| 7. | Gerber HP, Ferrara N. Pharmacology and pharmacodynamics of bevacizumab as monotherapy or in combination with cytotoxic therapy in preclinical studies. Cancer Res. 2005;65:671-680. [PubMed] |