INTRODUCTION
Cytosolic Ca2+ is an important second messenger in virtually every type of cell. Ca2+ regulates a wide range of cell functions, including contraction, gene transcription, cell growth, differentiation, apoptosis, membrane fusion, ion channel activation, and secretion[1]. Moreover, Ca2+ generally regulates multiple activities within individual cells. Temporal Ca2+ signaling patterns, such as Ca2+ oscillations, and spatial signaling patterns, such as Ca2+ gradients and waves, permit Ca2+ to exhibit this differential regulation of cell function. Therefore, the manner in which cells organize their Ca2+ signals is critical for the types of signals they can produce, and thus is critical for the ways in which Ca2+ can regulate cell function. There are certain common mechanisms for generating Ca2+ signals among cell types, but each cell type has distinct features of signaling machinery as well. Here we will review the machinery that is responsible for Ca2+ signaling in cholangiocytes. We also will discuss in detail two Ca2+-mediated events in cholangiocytes, bicarbonate secretion and apoptosis. Finally, we will review emerging evidence that Ca2+ signaling plays an important role in the pathogenesis of diseases affecting the biliary tree and in the treatment of cholestatic disorders.
Ca2+ SIGNALING MACHINERY IN CHO-LANGIOCYTES
Cytosolic Ca2+ signals originate from two general sources, release from intracellular Ca2+ stores and influx across the plasma membrane. The initial component of Ca2+ signals in cholangiocytes and other epithelia is due entirely to Ca2+ release from intracellular stores, since this component is unchanged in Ca2+-free medium[2,3]. In contrast, the role of extracellular Ca2+ is to maintain cytosolic signals over longer time intervals and to replenish intracellular stores. This review will therefore focus on intracellular Ca2+ release mechanisms. There are two intracellular Ca2+ release channels that are principally responsible for Ca2+ release in nearly every cell type, the ryanodine receptor (RyR) and the inositol 1,4,5-trisphosphate (InsP3) receptor (InsP3R)[1]. The RyR is most heavily expressed in excitable cells such as myocytes and to a lesser extent in neurons. The RyR also is expressed in some nonexcitable cells, including certain polarized epithelia, such as pancreatic acinar cells[4]. The RyR is localized to sarcoplasmic or endoplasmic reticulum (ER), where most intracellular Ca2+ is stored. Activation of the receptor allows Ca2+ to be released from the ER into the cytosol. The RyR can be activated in several different ways, including through direct coupling with certain plasma membrane Ca2+ channels, via stimulation with the second messenger cyclic ADP-ribose, or by stimulation with Ca2+ itself (Ca2+-induced Ca2+ release). Many epithelia, including cholangiocytes[5] as well as hepatocytes[6], do not express the RyR.
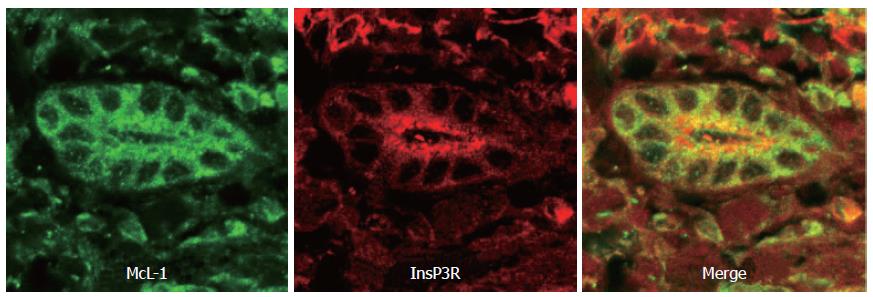
The InsP3R is expressed in virtually every cell type, is the predominant Ca2+ release channel in epithelia, and is the only intracellular Ca2+ release channel in cholangiocytes[5]. Like the RyR, the InsP3R is expressed in the ER. The endogenous ligand for the InsP3R is InsP3, which is formed from phospholipase C (PLC)-induced hydrolysis of phosphatidyl inositol bisphosphate (PIP2)[7]. This biochemical pathway can be activated by stimulation of either G protein-coupled receptors or receptor tyrosine kinases[1]. Therefore, stimulation of cholangiocytes with certain hormones or growth factors results in InsP3-mediated increases in cytosolic Ca2+. Hormone-induced Ca2+ signaling is mediated entirely by InsP3 in cholangiocytes, since Ca2+ signaling is blocked by microinjection of the InsP3R inhibitor heparin[2]. There are three InsP3R isoforms, each of which has distinct biophysical properties[8,9]. These properties result in different effects on Ca2+ signaling. For example, in DT40 cells engineered to express only a single InsP3R isoform, activation of the type I InsP3R results in transient Ca2+ oscillations, while the type II InsP3R induces sustained oscillations, and the type III isoform results in a single, transient increase in Ca2+[10]. Similarly, in CHO cells in which expression of each one of the three isoforms has been silenced, ATP-induced Ca2+ signals are preferentially converted from oscillations to either sustained or transient increases in Ca2+[11]. Many cell types express multiple InsP3R isoforms, and some cells[12,13], including cholangiocytes[5], express all three isoforms. Even among cell types that express all three isoforms, there is considerable variability in terms of how much of each isoform is expressed, and in terms of the subcellular region in which each isoform is expressed. In cholangiocytes, the type III InsP3R accounts for about 80% of InsP3Rs, while the types I and II isoforms each account for about 10%. Moreover, the type III InsP3R is most concentrated in the apical region (Figure 1), while the other two isoforms are not distributed in a polarized fashion[5]. Hormone-induced Ca2+ signals in cholangiocytes begin as apical-to-basal Ca2+ waves[5], similar to what is observed in other polarized epithelia[6,14]. In cholangiocytes, this polarized Ca2+ signaling pattern is likely due to the apical concentration of the type III InsP3R, although it is not clear if this is because the receptor has properties that permit it to trigger Ca2+ waves[8], or simply because of the increased InsP3R concentration in the apical region[15]. Ca2+ signals also can spread among neighboring cholangiocytes. This effect is mediated by gap junctions in most epithelia[16], including cholangiocytes[17]. The predominant gap junction protein in cholangiocytes is connexin 43, although connexin 32 is expressed in these cells to a lesser extent[17]. Permeability of cholangiocyte gap junctions is decreased by protein kinases A and C[17], which provides additional ways in which Ca2+ signals can be modulated in intact bile ducts.
Figure 1 Distribution of the InsP3 receptor and Mcl-1 in human bile ducts.
This confocal immunofluorescence image was obtained from a paraffin-embedded biopsy specimen from a normal liver. The specimen was double-labeled with antibodies against Mcl-1 (green) and the type III InsP3R (red). Note that the Mcl-1 is distributed diffusely throughout the cytosol, while the InsP3R is most concentrated in the apical region.
INTERCELLULAR MESSENGERS THAT ACT THROUGH Ca2+
Several intercellular messengers have been identified that increase cytosolic Ca2+ in cholangiocytes. Each messenger molecule interacts with specific G protein-coupled receptors that increase Ca2+via activation of PLC and formation of InsP3. Acetylcholine (ACh) increases Ca2+ by stimulation of M3 muscarinic receptors, which have been identified on cholangiocytes by RT-PCR, immunoblot, and immunochemistry[18]. These receptors are expressed on the basolateral membrane[2]. ACh-induced increases in Ca2+ can have both indirect and direct effects on downstream signaling pathways. ACh-induced increases in Ca2+ activate calcineurin, which potentiates increases in cAMP that are induced by secretin[18]. Stimulation of cholangiocytes with ACh can result in a sustained, transient, or oscillatory increase in cytosolic Ca2+[2]. The likelihood that a particular pattern of Ca2+ signal will be elicited is not dose-dependent, though. ATP also increases Ca2+ in cholangiocytes[2]. Like ACh, ATP can induce a sustained, transient, or oscillatory increase in cytosolic Ca2+. Unlike ACh, lower concentrations of ATP predominantly induce Ca2+ oscillations, while higher concentrations induce single (either sustained or transient) increases in Ca2+[2]. RT-PCR evidence suggests that cholangiocytes express P2Y1, P2Y2, P2Y4, P2Y6, and P2X4 nucleotide receptors[19]. Pharmacologic evidence using microperfused bile duct segments isolated form rat liver suggests that each of the four P2Y subtypes are expressed on the apical membrane of cholangiocytes. P2Y receptors also are expressed on the basolateral membrane, but the actions of ATP in that region are attenuated by ecto-nucleotidases expressed at that membrane[19] or by nearby portal fibroblasts[20]. Pharmacological studies suggest that there is little or no functional expression of P2X receptors in cholangiocytes[19]. These findings suggest that the principal route for signaling to cholangocytes through ATP is via bile. ATP may be released from cholangiocytes to signal in an autocrine or paracrine fashion[21], and this pathway is important for cell volume regulation[22]. ATP also can be secreted into bile from hepatocytes[21,23], which provides a pathway through which hepatocytes can signal to cholangiocytes[24]. The manner in which this signaling pathway is regulated has not been established, but certain bile acids appear to activate this pathway in order to stimulate ductular bicarbonate secretion[23].
Cholangiocytes also express receptors for a number of neuropeptides that act through Ca2+ signaling. Cholangiocytes express both the serotonin 1a and 1b receptor on their basolateral membrane[25]. Stimulation of this receptor inhibits the bile duct proliferation that occurs following bile duct ligation. This is likely mediated through Ca2+ signaling, since receptor stimulation is associated with an increase in InsP3 and the effects on cholangiocyte proliferation are blocked by chelation of cytosolic Ca2+[25]. Work in cholangiocyte cell lines has shown that these cells also express CCK-B gastrin receptors. As with serotonin receptors, stimulation of gastrin receptors inhibits cholangiocyte growth. The effect of gastrin also is blocked by chelation of cytosolic Ca2+[26]. Cholangiocytes express alpha-1 adrenergic receptors, primarily on their basolateral membrane. The alpha-1 adrenergic agonist phenylephrine increases secretin-stimulated ductal secretion, as measured in both intact rat liver and isolated rat bile duct units. Phenylephrine also potentiates the effects of secretin on cAMP production, and directly increases cytosolic Ca2+ and InsP3 in cholangiocytes[27]. Pharmacologic evidence suggests that cholangiocytes express D2 but not D1 or D3 dopamine receptors. Stimulation of these receptors inhibits rather than stimulates secretin-induced ductular secretion and cAMP production. This is thought to be because D2 receptor stimulation co-activates PKC-gamma, whereas gastrin receptor stimulation co-activates PKC-alpha[28]. Thus, although several types of receptors link to Ca2+ signaling in cholangiocytes, they can have distinct downstream actions because of differential effects on Ca2+ signaling patterns as well as because of differential activation of related signaling pathways.
REGULATION OF SECRETION VIA Ca2+
Cholangiocytes comprise less than 5% of normal liver, but play an important role in the process of bile secretion[29,30]. Cholangiocytes can be stimulated to increase bile flow by up to 50%[31], although cholangiocytes typically affect the pH rather than the volume of bile[32]. Two parallel signaling pathways exist to regulate ductular bicarbonate secretion, which are mediated by either cAMP or Ca2+. Evidence in isolated cholangiocytes and bile duct units suggests that cAMP stimulates chloride secretion via the cystic fibrosis transmembrane conductance regulator (CFTR), and that bicarbonate secretion then occurs via an associated chloride-bicarbonate exchanger. Some evidence in isolated cell systems suggests that Ca2+ analogously stimulates chloride secretion via a Ca2+-activated chloride channel, and that bicarbonate secretion then occurs via an associated chloride-bicarbonate exchanger[33]. Alternative evidence instead suggests that Ca2+ stimulates ductular secretion by calcineurin-mediated potentiation of the cAMP pathway[18]. The mechanism of ductular secretion has also been investigated in the intact liver, by bivascular perfusion of the isolated liver via both the hepatic artery and the portal vein[32]. This bivascular perfusion model is necessary to use because the blood supply of cholangiocytes is derived via the hepatic artery in normal liver. Thus, for example, the effects of secretin on ductular secretion can be readily detected when this hormone is infused via the hepatic artery, but are difficult to detect when infused via the portal vein[32]. These studies show that ductular bicarbonate secretion induced by the Ca2+ agonist ACh depends upon both chloride channels and chloride-bicarbonate exchange. This observation supports the idea that Ca2+-mediated bicarbonate secretion results from serial activation of chloride channels, and then chloride-bicarbonate exchange. However, bivascular perfusion studies show that cAMP-mediated bicarbonate secretion depends upon chloride channels but not chloride-bicarbonate exchange[32]. This observation supports the idea that ductular bicarbonate secretion induced by cAMP may occur directly via CFTR, similar to what has been described in intestine[34,35].
REGULATION OF APOPTOSIS VIA Ca2+
Apoptosis in all cell types is regulated in part through Ca2+ signaling pathways, and there are special considerations in cholangiocytes. Apoptotic pathways are modulated through mitochondrial permeabilization, which in turn is regulated through Ca2+ signaling. The interrelationship between mitochondria and Ca2+ signaling results from the close proximity between mitochondria and InsP3Rs[36]. As a result of this physical association, mitochondria are subjected to more intense increases in Ca2+ than typically occur in cytosol[36]. Excessive increases in mitochondrial Ca2+ result in increases in mitochondrial permeability that are associated with leakage of cytochrome c into the cytosol, which leads to apoptosis[37]. Cytochrome c released from mitochondria binds directly to nearby InsP3Rs, which potentiates release of Ca2+ from the receptor[38]. This leads to a positive feedback loop that further increases mitochondrial permeability and promotes apoptosis[38]. On the other hand, the anti-apoptotic Bcl-2 family members Bcl-xL and Bcl-2 inhibit apoptosis by inhibiting InsP3-mediated Ca2+ release. Bcl-xL decreases expression of InsP3R, which reduces InsP3-mediated Ca2+ release in order to protect against apoptosis[39]. Bcl-2 itself increases leakage of Ca2+ from the ER, which also has the net effect of decreasing Ca2+ signals in both the cytosol and mitochondria[40]. Mcl-1 is the principal Bcl-2 family member in cholangiocytes[41] (Figure 1). Like other Bcl-2 family members, it appears to inhibit apoptosis through effects on Ca2+ signaling. Unlike Bcl-xL or Bcl-2, however, Mcl-1 does not affect either InsP3R expression or ER Ca2+ stores[42]. Instead, Mcl-1 inhibits Ca2+ signaling directly within mitochondria[42].
All three isoforms of the InsP3R can induce apoptosis[11]. However, each isoform has a different propensity to induce apoptosis[11]. Recent evidence suggests that this may be due in part to their differential distribution relative to mitochondria. Specifically, in cells that co-express each InsP3R isoform, the type III InsP3R is most effective in transmitting Ca2+ signals to mitochondira as well as in inducing apoptosis[11]. Furthermore, the type III isoform colocalizes most strongly with mitochondria at the light level[11]. Thus, the type III InsP3R has a particular propensity to form signaling microdomains with mitochondria. The type III InsP3R is most concentrated in the apical region of cholangiocytes[5], as described above, but little is known about the factors that regulate this subcellular targeting. It also remains unknown whether apical, type III InsP3Rs associate with mitochondria in cholangiocytes, or whether such an association is needed to mediate apoptosis in bile ducts.
Ca2+ SIGNALING IN HEALTH AND DISEASE
Ca2+ signaling is universal among cells, so one might expect altered Ca2+ signaling to play an important role in pathogenesis of disease. In fact, the spectrum of human diseases that had been found to result from alterations in Ca2+ signaling has been remarkably limited. These have included a mutation in the RyR, which results in malignant hyperthermia[43]; mutations of the Ca2+-ATPase pump, which are associated with deafness[44]; and an animal model of loss of the types II and III InsP3Rs, which together results in pancreatic insufficiency and failure to thrive[45]. However, it now appears that loss of InsP3Rs is a common event in human cholestatic disorders. Specifically, expression of the type III InsP3R is decreased in the cholangiocytes of patients with primary biliary cirrhosis, sclerosing cholangitis, biliary atresia, and ductular obstruction[46]. This loss of InsP3R expression also occurs in animal models of cholestasis, where it is associated with impaired Ca2+ signaling, as well as impaired Ca2+-induced secretion in cholangiocytes[46]. However, cAMP-mediated secretion is maintained in cholangiocytes despite the loss of InsP3Rs. Interestingly, the loss of InsP3Rs that is seen in patients with cholangiopathies is not seen in patients with hepatitis C viral infections, even though such infections are associated with inflammation in the region of the portal triad[46]. This suggests that loss of InsP3Rs from cholangiocytes is not merely a nonspecific effect of local inflammation. Together, these findings suggest that Ca2+ signaling pathways are necessary for ductular secretion as it occurs in vivo, and that loss of InsP3Rs results in cholestasis, although direct support for this hypothesis is lacking.
Just as impaired Ca2+ signaling in cholangiocytes is associated with the development of cholestasis, stimulation of Ca2+ signals may be beneficial for the treatment of cholestasis. Ursodeoxycholic acid (UDCA) and its taurine conjugate (TUDCA) are of therapeutic benefit in a range of cholestatic disorders[47]. Recent evidence suggests that these but not other bile acids selectively induce hepatocytes to secrete ATP into bile[23]. In addition, luminal perfusion of isolated intrahepatic bile duct segments with ATP increases Ca2+ in cholangiocytes and stimulates ductular bicarbonate secretion, while perfusion with TUDCA does not[23]. These findings suggest that UDCA and TUDCA may promote ductular secretion through a paracrine signaling pathway whereby hepatocytes release ATP to activate apical purinergic receptors on cholangiocytes, which link to Ca2+-mediated secretion of chloride and bicarbonate into bile. Further work is needed to understand how these bile acids induce hepatocytes to secrete ATP, and to demonstrate that this action is in part responsible for their therapeutic effects.
CONCLUSION
Ca2+ signaling is a ubiquitous mechanism for regulation of cell function. A range of neuroendocrine and paracrine messenger molecules exert their effects on cholangiocytes through Ca2+ signaling. It is already established that these effects include regulation of bicarbonate secretion and regulation of apoptosis. By extension from what has been shown in other cell types, it is likely that Ca2+ signaling also regulates other aspects of ductular secretion, including fluid secretion and vesicular targeting and exocytosis, as well as other aspects of cell proliferation, including gene transcription and progression through the cell cycle. Future areas of investigation are likely to more fully identify the range of actions of Ca2+ in cholangiocytes. Such information may in turn reveal specific ways in which impaired Ca2+ signaling leads to cholangiopathies and related disease states, which then may lead to targeted therapies that are based on correcting such impairments.
S- Editor Wang J E- Editor Liu WF