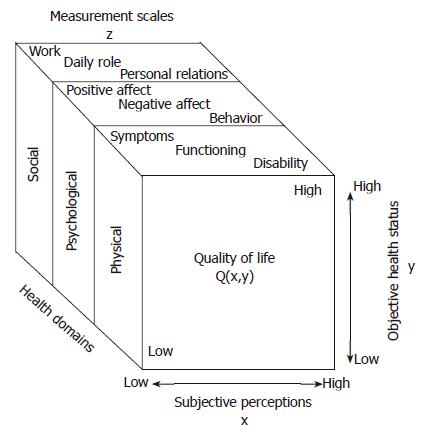
DESCRIPTION OF QUALITY OF LIFE
The assessment of quality of life (QOL) is a relatively new facet of health services research in surgery. Traditionally, surgeons measured success or failure based on perioperative morbidity and mortality. However, as technology has improved surgeons’ ability to intervene safely in patients’ lives, researchers have been compelled to determine whether improvement in survival translates into improvement in QOL. QOL is a nonspecific term that attempts to define the overall state of a person’s life. There are subjective and objective components to the QOL specific to one person. For example, two people with the same health and economic status could perceive their respective qualities of life very differently. Discrepancies in QOL are influenced by the social and psychological status of the individual. Research tools have been developed to quantify both subjective and objective evaluations of quality of life (Q) in order to obtain a healthcare-related value of QOL. Measurement scales are based on social, somatic, psychological, and physical domains to obtain a value (Z). To be validated and used in trials, Z must be shown to equal Q (Figure 1)[1].
Figure 1 To measure QOL, social, psychological and physical domains of illness are assessed using measurement scales (z).
QOL (Q) is dependent on both subjective and objective items. For a questionnaire to be validated, Z must equal Q. (Reprinted with permission from Testa et al N Engl J Med 334: 835-840, 1996).
QOL studies have increased rapidly in the medical literature, as demonstrated by the number of citations on Medline. In 2004, a keyword search on “quality of life” identified 5584 articles, whereas 20 years earlier (1984), only 45 citations were elicited. QOL trials can discriminate between therapies with similar rates of morbidity and mortality, such as diverting ostomy or bowel resection with primary anastomosis in colon cancer, or compare different treatment modalities, such as medicine versus surgery. Assessment of QOL outcomes requires accurate gathering of information, use of pertinent measurement tools, and appropriate follow-up. A structured QOL questionnaire consists of a number of items in the form of questions or statements that draw on various dimensions of QOL . The form used to assess these subjective and objective data must be validated and then filled out either by the patient, a proxy, or by interview (telephone or face-to-face). The telephone interview limits follow-up to people who do not have hearing impairments and who have a telephone. Self-administered questionnaires give patients the opportunity to address problems they might otherwise be reluctant to discuss openly with an interviewer, but there is an increased risk of misinterpretation. Face-to-face interviews decrease misinterpretation but might fail to represent aspects of QOL people are embarrassed to discuss. Survey answers are assigned a score; qualitative information is converted into quantitative data, such that standardized scores are inclusive of all represented dimensions within the instrument[2].
Instruments calculate QOL in a generic or specific manner. Generic questionnaires provide an overall assessment of QOL that is not matched for a certain population or disease state. A popular, validated example of a generic QOL instrument is the Short Form-36 (SF-36). The questionnaire includes 36 items that measure eight dimensions of health on multi-item scales, including physical function, emotional function, physical problems, emotional problems, pain, general health, vitality, and social function. Sample questions include “Does your health now limit you in vigorous activities, moderate activities, lifting or carrying groceries” and “Have you felt downhearted and blue” There is also a single-item scale measuring changes in health during the previous year. The scoring scale ranges from 0 to 100, with 0 equal to death and 100 equal to perfect health. On the SF-36 scale, higher scores imply better health[3]. The SF-36 is useful for assessing health in a global manner; for example, the SF-36 has been used around the world to assess QOL in patients with a variety of different chronic diseases[4]. However, the SF-36 is often not sensitive enough to detect small differences in specific treatment groups with unique health care disabilities. Specific health-care instruments are tailored to detect these small differences in QOL when treatment groups are more homogeneous. These questionnaires are specific to a type of disease, population, domain, or symptom[5].
In 1980, the European Organization for Research and Treatment of Cancer (EORTC) started a research program to develop an integrated approach for QOL evaluation in patients with cancer participating in international clinical trials. The goal was to develop a core questionnaire incorporating a wide range of physical, emotional, and social health issues relevant to a broad range of cancer patients, irrespective of specific diagnoses, supplemented with diagnosis-specific (i.e. esophageal, pancreatic, and colorectal cancers) and/or treatment-specific modules. Currently, the EORTC QLQ-C30 is the most widely used disease-specific instrument for the assessment of QOL in cancer patients worldwide. The 30-item questionnaire incorporates five functional scales (i.e. physical, role/daily activities, cognitive, emotional, and social), three symptom scales (i.e. fatigue, pain, nausea and vomiting), and a global health and QOL scale. The single items assess commonly-reported symptoms of cancer patients (i.e. dyspnea, appetite loss, sleep disturbance, constipation, and diarrhea), as well as the perceived financial impact of the disease and treatment[6].
It is important to distinguish between the measurement of direct and indirect costs accrued by patients during the diagnosis, treatment, and surveillance phases of their diseases. Direct health care costs include expenses accrued during visits to physicians, as well as the costs of prescription medications, physical therapy, hospitalization, operative procedures, laboratory, and radiological tests. Indirect costs include the value of production lost to illness-related absence, such as the number of days absent from (paid and unpaid) work and days lost from housekeeping and other daily activities. Currently, large administrative databases compiled by insurance companies, hospitals, and other healthcare institutions are the most reliable source for cost estimation, but they do not include most indirect costs. Estimation of the financial impact of disease on patients is a difficult task. The QLQ-C30 addresses the financial impact of disease by asking “Has your physical condition or medical treatment caused you financial difficulties” However, other studies have attempted to further quantify the financial impact in a more specific manner. Goossens et al[7]. studied the use of a patient cost diary to evaluate all indirect costs in 205 patients with fibromyalgia and low back pain over a two-year period. They found that when the diary was used in conjunction with periodic interviews, there was an accurate estimate of direct and indirect costs, as well as good patient compliance completing the diary. Additional studies have shown that the cost of disease treatment can be collected prospectively to assess for differences in treatment arms using a Collection of Indirect and Nonmedical Direct Costs (COIN) form[8]. In order to fully evaluate the impact of financial strain on QOL, these forms can be used in conjunction with the QLQ-30.
ESOPHAGEAL CANCER
Esophageal cancer is the second most common solid intrathoracic malignancy in the U.S., after lung cancer. The National Cancer Institute (NCI) estimates that 14 520 Americans are diagnosed annually with esophageal cancer, and 13 570 die of their disease.(http://www.cancer.gov) Management of esophageal cancer depends on the stage of disease at presentation and the general health of the patient; treatment options include surgery, chemotherapy, radiation therapy, multimodality therapy, or palliative care. The esophageal cancer model is a good example of how a generic questionnaire was developed and then refined to obtain a specific QOL instrument that targets patients with esophageal cancer and their disease-specific symptoms. One of the first studies to examine QOL in patients with esophageal cancer used the EORTC QLQ-30 instrument, in combination with a linear dysphagia scale, to assess for disease-specific complaints. This study by Blazeby in 1995 followed 59 patients (33 post-esophagectomy and 26 post-palliative care) for a median of 16 wk. Overall, patients undergoing palliation had lower QOL scores. However, the palliation group was heterogeneous, in that some patients received esophageal stents and pain medicine, while others received only pain medicine. Symptoms of dysphagia captured on the linear dysphagia scale did not correlate well with global QOL scores acquired on the QLQ-30. The authors concluded that the QLQ-30 is a valid instrument for assessing outcomes after treatment only if an additional disease-specific measure was added to identify dysphagia, an important QOL outcome for patients with esophageal cancer[9].
As a result of this study, esophageal patients and medical experts were consulted to reform and create a new questionnaire. The EORTC QOL Group developed a disease-specific module (QLQ-OES24) for patients with esophageal cancer undergoing surgery, chemotherapy, radiotherapy, and/or endoscopic treatment[10]. Using the EORTC QLQC30 and the dysphagia scale from the EORTC QLQ-OES24, QOL was followed this time in a prospective longitudinal study of patients with esophageal cancer[11]. Blazeby et al[12]. followed patients who underwent esophagectomy (n = 55) or palliative treatment with chemotherapy ± stenting (n = 37). QOL scores were obtained before treatment and at regular intervals. Median scores were calculated for patients surviving more than two years after esophagectomy (n = 17), for patients surviving less than two years (n = 38), and for patients undergoing palliative treatment (n = 37). Six weeks after esophagectomy, patients reported worse functional, symptom, and global QOL scores than before treatment. In patients that survived at least two years from the time of treatment, QOL scores returned to pre-operative levels within nine months, but patients who died less than two years after surgery never regained their pre-operative QOL. In both surgery groups, dysphagia improved, and the improvement was maintained until death, or for the duration of the study. Patients undergoing palliative treatment had more advanced disease and were more physically compromised from the beginning of the study. Palliative intervention allowed these patients to maintain their compromised QOL until death. The study demonstrated that esophagectomy had a negative impact on QOL in the short-term. Patients surviving less than two years had an improvement in dysphagia, but not in overall QOL. The authors concluded that if the subset of patients that would not survive two years could be identified pre-operatively, esophagectomy should be avoided and palliation and/or stenting should be recommended instead. In the following years, the QLQ OES24 module was refined to more clearly elicit symptoms of dysphagia and other problems with eating, as they are especially important for patients with esophageal cancer. With further analysis, items with poor convergence were eliminated. Ultimately, the module was refined to four scales and six single items; the QLQ-OES18 is now the prevailing validated disease-specific QOL measure for esophageal cancer (Figure 2). It should be used in combination with the QLQ-C30.
Figure 2 The EORTC QLQ-18 is a specific module for esophageal cancer.
Patients complete the questionnaire based on QOL symptoms experienced within the last week. (Reprinted with permission from the EORTC.)
The Rotterdam Symptom Checklist (RSCL), developed in 1990, collects information about physical symptoms after surgery specific to esophageal cancer, including “dysphagia, loss of taste, weight loss, early satiety, blown-up feeling, hoarseness, pain behind the chest bone, food not going down, and nocturnal coughing[13]. A recent study by de Boer et al[14]. used the validated RSCL in combination with the Medical Outcomes Study Short Form-20 to assess QOL. Patients were randomized to receive a transhiatal (THE) or transthoracic esophagectomy and were followed for three years. Three months after surgery, patients in the THE esophagectomy group (n = 96) reported fewer physical symptoms (P = 0.01) and better activity levels (P < 0.01) than patients in the transthoracic group (n = 103). However, this difference was not sustained, and at longer follow-up there was no significant difference between the two treatment groups.
Recent trials have shown that clinical outcomes from esphagectomy can be improved by administering neoadjuvant chemotherapy[15]. In 2005, Blazeby examined the effects of neoadjuvant chemo-and radiation therapy on QOL in patients with esophageal cancer. Data were collected using the EORTC QLQ-30 in tandem with the cancer-specific QLQ-OES18. Patients receiving neo-adjuvant chemotherapy (n = 48) or chemoradiotherapy (n = 34) were noted to have a decline in QOL during treatment that was restored by the time of surgery. Patients who received neoadjuvant chemo-radiation had a relative improvement in post-operative nausea, dysphagia, and emesis compared to patients who underwent esophagectomy alone (P < 0.01). The authors concluded that neoadjuvant therapy improves disease-specific survival and potentially improves postoperative QOL[16].
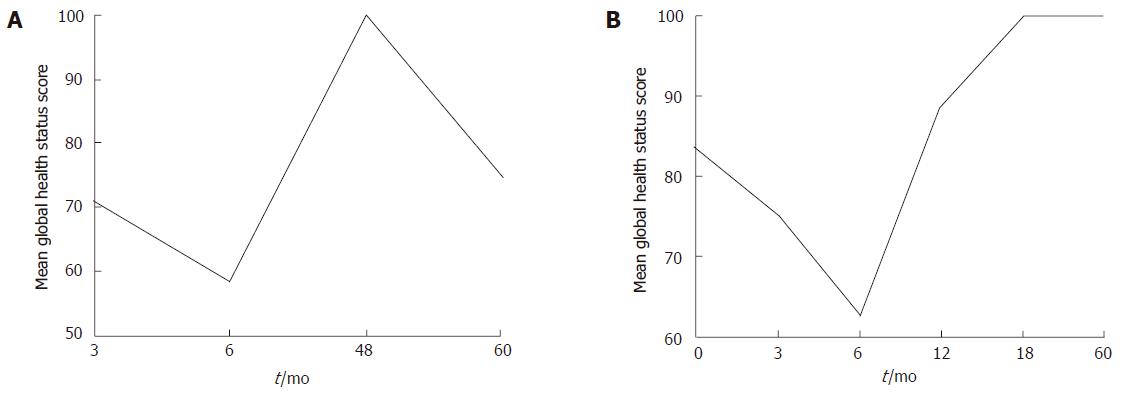
At Yale, we are conducting a prospective cohort study measuring QOL in patients with esophageal adenocarcinoma who undergo THE with or without neoadjuvant chemo-and/or radiation-therapy. The specific aim of the study is to better select patients who will benefit from this type of surgery by identifying demographic and clinical predictors of QOL following THE. The EORTC QLQ-OES18 is administered prior to surgery and then at regular intervals until death or for five years. Preliminary results for the first 21 patients demonstrate a dramatic decrease in global QOL scores in the first six months following surgery, with a gradual improvement in the following year (unpublished results). When comparing age groups following THE, the global QOL score of patients over age 70 years improves after the first six months, and then declines after two years (Figure 3A). In contrast, patients 55 years or younger improve their QOL to pre-operative levels by six months, and these improvements appear to be durable (Figure 3B) The trends in specific symptom scores (i.e. fatigue, nausea and vomiting, pain, and loss of appetite) are variable: they rise and fall during the first year, ultimately declining steadily by the fourth post-operative year. Dysphagia and reflux scores, with a range of 0 (no dysphagia or reflux) to 100 (complete dysphagia or reflux), plateau within the first year; the dysphagia score drops to 0, and the reflux score peaks (mean score, 45) at 18 mo. Functional scores (i.e. physical, emotional, and social functioning) nadir six months following surgery and then rebound to peak at four years; however, scores steadily decline by the fifth year after surgery.
Figure 3 A: QOL after THE in patients with esophageal cancer ≥ 70 years of age.
QOL declines sharply for six months and then improves, but the recovery is not durable; B: QOL after THE in patients with esophageal cancer ≤ 55 years of age. QOL declines for six months, but then improves in a more sustained fashion.
More patients must be enrolled to better characterize the relative influences of such demographic and clinical factors as patient gender, race, tumor stage, comorbidities, and the use of neo-adjuvant and/or adjuvant treatment. Still, from our early data, we would conclude that candidates for THE should be counseled pre-operatively about the effects of surgery on QOL. The association between age and QOL outcomes should be considered when advising patients to undergo THE; that is, patients over age 70 should have a life expectancy of at least six to twelve months post-operatively to warrant the profound effects of THE on QOL.
COLON AND RECTAL CANCER
Colorectal cancer is the third most common cancer in the United States. The American Cancer Society estimates that in 2005, there will be 148 300 new cases of colon and rectal cancer, and 56 290 people will die of their disease (http://www.cancer.org). Generally, surgery is the initial therapy for patients with colon and rectal cancers. There are two prevailing validated QOL assessment tools for colon and rectal cancer: the Functional Assessment of Cancer Therapy-Colorectal (FACT-C) and the EORTC QLQCR 38, which like the QLQ-OES18 for esophageal cancer, is designed for use with the EORTC QLQ-C30. These instruments have been used in patients undergoing palliative or surgical treatment, regardless of the stage of their malignancy. The EORTC QLQ-CR 38 is a validated, reliable instrument that consists of 38 items inquiring about symptoms specific to colorectal disease and treatment, such as body image, sexuality, urinary problems, defecation or stoma, and future outlook on life[17]. The FACT-C was developed around the same time as the EORTC QLQ-CR 38. It is a shorter questionnaire, composed of 36 items from the core Functional Assessment of Cancer Therapy (FACT-G) merged with the colon and rectal specific items (CCS). These items were developed from interviews with colorectal patients and caregivers. They include two stoma-related questions. In 1999, Ward et al[18]. tested the FACT-C in English and Spanish-speaking patients with colorectal disease and found them to be reliable and valid. The authors concluded that the FACT-C had good internal consistency, reliability, and concurrent validity by addressing specific concerns related to colorectal cancer. In addition, in the Spanish population, it elicited subtle differences in QOL based upon extent of disease.
In a recent study by Rauch et al[20], 121 patients who received surgery for rectal cancer and who survived at least two years without evidence of disease recurrence were identified. These rectal cancer survivors were mailed three questionnaires: the EORTC QLQ-C30, the QLQ-CR38, and the Duke questionnaire. The Duke health profile is a 17-item validated instrument to assess QOL, but it is not specific for colorectal cancer[18]. The study patients reported less pain when compared to historical controls drawn from the general German and Norwegian populations (P = 0.002). Stoma patients reported better social functioning than did non-stoma patients (P = 0.005), with less anxiety (P = 0.008) and higher self-esteem (P = 0.0002). However, this was only detected on the Duke health profile, whereas the QLQ-30 did not demonstrate any difference. It is unclear whether the more generic Duke profile captured some confounding effect on QOL (i.e. the positive effects of successful medical treatment), or if the EORTC module was too specific and failed to demonstrate real differences in QOL[19].
A recent study by Holloway et al[21]. from the University of Texas examined QOL as a predictor of post-operative length of stay (LOS) in 70 patients with colon and rectal cancers using the FACT-C module[20]. Patients completed the FACT-C questionnaire before treatment. LOS for patients scoring in the lowest quartile on the FACT-C in the pre-operative setting was compared with LOS for patients in the remaining quartiles. Patients in the lowest quartile of FACT-C scores had significantly longer LOS. Specifically, lower pre-operative FACT-C scores for physical well-being (9.1 vs 7.3 d; P = 0.04), functional well-being (9.6 vs 7.1 d; P = 0.006), and colon cancer concerns (9.5 vs 7.1 d; P = 0.01) correlated with increased LOS. Advanced patient age and surgical morbidity also predicted longer LOS, but additional factors such as race, nutritional status, stage of disease, and relative training of the operating surgeon did not appear to affect LOS. Courneya et al[22]. from Canada utilized the FACT-C on QOL in a prospective randomized-controlled trial to measure the effect of exercise on QOL after any type of colorectal surgery. Patients were assigned in a 2:1 ratio to either an exercise (n = 69) or control (n = 33) group. Exercise consisted of a home cardiovascular workout lasting 20-30 min, 3-5 times/wk; subjects had to reach at least 65% of their predicted target heart rate. Compliance was not good; therefore, analysis was limited to a comparison between patients who increased physical fitness to patients who had no change or decreased fitness. There was a significant improvement in QOL based on FACT-C scores between patients with increased physical fitness when compared to patients who did not increase physical fitness[21].
In 2005, Jung Yoo et al[23]. administered the FACT-C to 98 patients with colorectal cancer at the time of any surgery for rectal cancer. Fifty-two patients completed the survey at one and six months post-operatively; 38% received a stoma as part of the cancer operation. Analysis of variance between FACT-C scores demonstrated that patients’ QOL was nadired one month after colectomy and returned to pre-operative levels six months later. This is an example of how QOL analysis can provide useful nformation for the patient regarding the projected post-operative course, which allows them to have some realistic expectations for recovery time and QOL[22].
PANCREATIC CANCER
Pancreatic cancer is the fourth leading cause of cancer-related death in males and the fifth leading cause in females in the Unites States (http://www.cancer.gov). It is usually diagnosed at an advanced stage, so survival is poor. There has been little change in overall incidence or mortality rates over the last three decades. Fewer than five percent of patients survive five years from the time of diagnosis. Whipple first described the modern pancreaticoduodenectomy (PD) in 1935. It remains the standard surgical treatment for adenocarcinoma of the head of the pancreas. Peri-operative mortality has diminished significantly, with experienced centers reporting rates of less than 5%. However, complications continue to be common and include pancreatic fistula, delayed gastric emptying, intra-abdominal abscess and hemorrhage, and anastomotic leak. Therefore, QOL studies in pancreatic cancer patients could play a significant role in determining appropriate treatment options. The EORTC QLQ-30 module was modified to identify symptoms that are unique to patients with pancreatic cancer. Over a two-year period, the EORTC study group on QOL performed literature searches, interviewed patients and health-care professionals, and identified 26 items related to pancreatic cancer that resulted in the QLQ-PAN-26 instrument[23]. A study by Shaw et al[25]. in 2005 examined long-term QOL following PD for benign and malignant disease[24]. The treatment group included 40 patients who underwent PD for benign and malignant disease. The benign group included patients with chronic pancreatitis, neuroendocrine tumors, pseudopapillary tumors and microcyst adenomas. The control group consisted of 58 patients who underwent cholecystectomy by the same surgeon. The two groups were matched for age and gender. Patients were followed for nine years. QOL was assessed using the EORTC QLQ-C30 and the QLQPAN26. While there was no significant difference in global health scores between the two groups, the QLQ-30 identified significant decreases in the post-operative functional scales (physical role, emotional role, and social) amongst the study PD group as compared with the control group. The malignant PD group reported the lowest level of physical and role functioning, greater weakness and concerns for future health, while the benign group had more social/financial difficulties, and upper gastrointestinal symptoms consistent with exocrine insufficiency. Upper gastrointestinal symptoms posed greater difficulties for patients who underwent total pancreatectomy including absolute dependence on pancreatic exocrine supplements and insulin-dependent diabetes. Patients after a PD had more diarrhea (P < 0.05) and nausea and vomiting (P < 0.05) than the matched patients after cholecystectomy. In part as a result of this study, patients are routinely placed on pancreatic enzyme supplements following PD at this research hospital.
A 2005 prospective longitudinal study by Nieveen van Dijkum et al[26]. assessed QOL in 114 patients following PD and a double-bypass procedure (hepaticojejunostomy and gastroenterostomy) for pancreatic and periampullary carcinoma[25]. The study employed the Medical Outcomes Study (MOS) 24 acute-phase questionnaire, the disease specific Gastrointestinal Quality of Life Index (GIQLI), and one question concerning overall QOL derived from the RSCL[13]. The GIQLI is an instrument developed in 1995 that has been validated in many patients with gastrointestinal diseases[26]. The questionnaires were administered over one year, or until death. A transient decline in physical (MOS score) and gastrointestinal (GIQLI score) functioning were observed at two weeks after surgery for both treatment groups. PD patients regained QOL within six weeks of surgery. Double bypass patients did not return to the pre-operative QOL until three months after surgery. There was a more pronounced decline in all QOL scales after double-bypass procedures as compared with PD. The authors concluded that PD maintains QOL in patients with resectable pancreatic cancer. Palliative double bypass procedures have a beneficial effect on QOL in patients with unresectable pancreatic cancer that are expected to survive at least three months[27].
In conclusion, QOL studies seek to quantify symptoms and perceptions patients have in a generic- or disease-specific manner. Validating subjective and objective data has been labor-intensive; a large body of the work to date has been initiated by the EORTC. The EORTC QLQ-C30 has been used to measure QOL among patients with cancer in a reliable and reproducible fashion. However, it is important to include specific modifiers relevant for different disease processes. For example, dysphagia is measured in esophageal cancer, bowel habits in colorectal cancer, and bile duct obstruction in pancreatic cancer. Historically, post-operative morbidity and mortality have been the barometers used to assess clinical outcomes from different surgical interventions. Interestingly, economic outcomes, such as hospital charges and length of stay have also been reported. QOL is yet another outcome measure that should be collected when gauging the overall appropriateness of a surgical intervention. QOL information should be conveyed to patients in the pre-operative setting, so that they can make more informed decisions regarding their healthcare. We believe future research and clinical trials in surgery options in patients should routinely include analysis of QOL as an outcome measure.
S- Editor Pan BR E- Editor Bai SH