Published online May 21, 2006. doi: 10.3748/wjg.v12.i19.3044
Revised: January 3, 2006
Accepted: January 9, 2006
Published online: May 21, 2006
AIM: To evaluate serum TIMP-1 level and the correlation between TIMP-1 expression and liver fibrosis in immune-induced and CCL4-induced liver fibrosis models in rats.
METHODS: Immune-induced and CCL4-induced liver fibrosis models were established by dexamethasone (0.01 mg) and CCL4 respectively. Serum TIMP-1 level was detected with ELISA, while histopathological grade of liver biopsy was evaluated. Spearman rank-correlation test was used to analyse the difference of the correlation between the TIMP-1 expression and hepatic fibrosis in the two fibrosis models. Furthermore, in situ hybridization was used to determine the expression difference of TIMP-1 mRNA in the two models.
RESULTS: Positive correlation existed between serum TIMP-1 level of immune induced group and the histopathological stages of fibrosis liver of corresponding rats (Spearman rank-correlation test, rs = 0.812, P < 0.05), and the positive in situ hybridization signal of TIMP-1 mRNA was strong. In CCL4-induced liver fibrosis model, the correlation between the serum TIMP-1 level and the severity of hepatic fibrosis was not statistically significant(Spearman rank-correlation test, rs = 0.229, P > 0.05). And compared with immune-induced model, the positive in situ hybridization signal of TIMP-1 mRNA was weaker, while the expression variation was higher in hepatic fibrosis of the same severity.
CONCLUSION: The correlations between TIMP-1 expression and liver fibrosis in two rat liver fibrosis models are different. In immune-induced model, serum TIMP-1 level could reflect the severity of liver fibrosis, while in CCL4-induced model, the correlation between the serum TIMP-1 level and the severity of hepatic fibrosis was not statistically significant.
- Citation: Nie QH, Zhang YF, Xie YM, Luo XD, Shao B, Li J, Zhou YX. Correlation between TIMP-1 expression and liver fibrosis in two rat liver fibrosis models. World J Gastroenterol 2006; 12(19): 3044-3049
- URL: https://www.wjgnet.com/1007-9327/full/v12/i19/3044.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i19.3044
Hepatic fibrosis is a pathological process with the net deposition of extracellular matrix (ECM) proteins. The change of ECM is mainly regulated by matrix metalloproteinases (MMPs), which are a family of proteolytic enzymes that are capable of degrading the ECM[1-3]. The activity of MMPs is tightly regulated by the amount of active protein and the concentration of specific inhibitors, called tissue inhibitors of metalloproteinases (TIMPs)[4,5]. Extensive studies have identified that TIMPs play a key role in the progression of fibrosis. TIMP-1 is the first discovered tissue inhibitor of TIMPs in liver which can inhibit most interstitial collagenases and MMP-9. Murawaki et al revealed that serum level of TIMP-1 could reflect the change of liver TIMP-1 in patients with chronic liver disease and liver TIMP-1 concentration increases with progression of the liver disease[6-9]. So, TIMP-1 has been considered as a useful diagnostic index of hepatic fibrosis[10-12]. Our previous studies[13-15] also concentrated on the establishment of the new hepatic fibrosis diagnostic index. Interestingly, in animal experiments we found the expression of TIMP-1 in liver tissue has remarkable difference in different rat liver fibrosis models[13,14]. In the present experiment, the serum TIMP-1 level of experimental rats of liver fibrosis model was detected with ELISA and association with histopathological grading of fibrosis liver was analyzed. Furthermore, in situ hybridization was used to analyze the expression difference of TIMP-1 mRNA between the two models.
One hundred and twenty adult female Wistar rats (provided by Experimental Animal Center of Fourth Military Medical University, Xi’an, China) weighing 150-180 g, fed with common stuff and water, were employed in the study (Approval for this project was obtained from the Institutional Review Board at Fourth Military Medical University). Immune-induced rat liver fibrosis model was established according to the method of Wang et al[16]. Dexamethasone (0.01 mg) was injected into the rats through coccygeal vein in one hour after the first and second injection of human serum albumin (HAS). For CCL4 group, 55 rats were injected with 400 mL/L CCL4 in peanut oil subcutaneously at a dose of 3 mL/kg twice (Monday and Thursday) weekly, totally 10 wk[17]. Ten normal rats were served as control group.
From the first Sunday in the process of model induction (for immune induced group, from the first coccygeal vein injection of HSA), 5 rats from each group were selected randomly on Sunday, until all rats were involved. Rats taken out from immune induced group on the first Sunday were numbered IW1A to IW1E (Immune-induced rat liver fibrosis model wk 1st A-E), while the rats from CCL4 induced group were numbered CW1A to CW1E (CCL4-induced rat liver fibrosis model wk 1st A-E). All rats were immediately sacrificed under narcosis after taken out. Following decapitation 2 mL blood was collected from each rat. Liver was quickly excised, and rinsed in ice-cold saline. Ten rats in control group were treated in the same way after 10 wks’ feeding. Blood specimen was centrifuged, and the serum was obtained, and stored at -70°C. Livers were cut into 1 mm × 1 mm × 1 mm blocks. Part of it was fixed in 40 g/L formaldehyde, and part was fixed in 25 g/L glutaraldehyde. All liver specimens were stored at -70°C. Specimens were treated all together after collected.
Liver sections were processed together for routine hematoxylin-eosin (HE) stain. Pathological diagnosis of each liver specimen was assessed and graded from 0 to VI by three pathologists in a blinded manner (fibrotic stage was determined when more than two pathologists reached the same diagnosis; if inconsistent results were reached among the three pathologists, the fourth determined the final diagnosis) according to the criteria described by Wang et al[18] (Table 1).
| Degree of fibrosis | Score |
| No fibrosis | 0 |
| Slight fibrosis expanding to some portal areas and central veins | I |
| Marked fibrosis expansion, but without portal to portal bridging | II |
| Fibrosis expanding to most portal areas with occasional portal to portal bridging | III |
| Pseudolobules formed and partly replacing the normal architecture of the liver lobules | IV |
| Occasional pseudolobules formed (incomplete cirrhosis) | V |
| Congested with pseudolobules, and between pseudolobules wide hyperplastic collagen fiber existed (complete cirrhosis) | VI |
Enzyme-linked immunosorbent assay (ELISA) was used to evaluate the serum TIMP-2 levell[19,20]. Mouse TIMP-2 monoclonal antibody was purchased from American Maxim Company (MAB-0283) and test was performed according to the protocol described previously[21]. The absorbance of serum TIMP-2 was determined at 450 nm with an ELISA reader (EL × 800, Bio-Tek Instruments. Inc, USA).
Paraffin sections were hybridized with digoxingenin-labelled cRNA probes as described by Wang et al[22]. The cRNA probe for TIMP-1 and the in situ hybridization kit were purchased from Boshide Biological Technology Limited Company, Wuhan, China (No MK1549). Expression of TIMP-2 mRNA was assessed quantitatively by image pattern analysis with gray scale scanning. Control was performed with normal liver tissue.
Specimens were routinely prepared for transmission electron microscopy (TEM) observation and examined under a JEOL JEM-100CXII electron microscope.
Spearman rank-correlation test, LSD-t and Student’s t test were used to analyze the results. P < 0.05 was taken as significant.
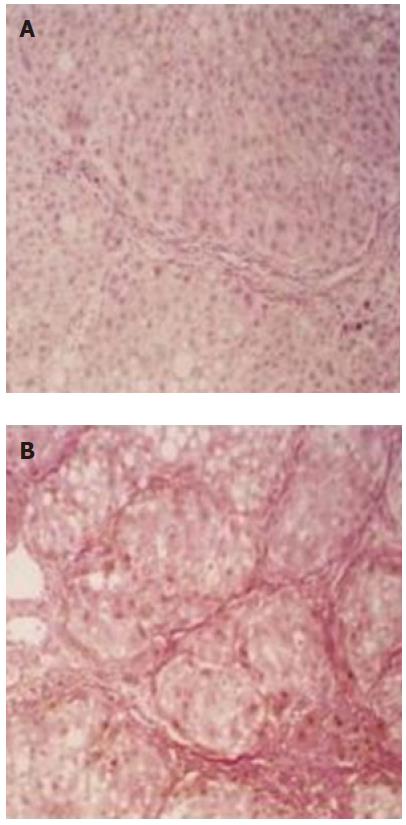
Distinct difference of histopathological changes presented between the liver fibrosis of immune group and CCL4 group. The liver fibrosis in immune group rat was characterized by portal fibrosis with marked ductular proliferation extending to fibrous septa (Figure 1A, specimen from IW10E). Pseudolobules formed and partly replaced the normal architecture of the liver lobules, but the fibrotic septum was small and sparse. In CCL4 group, prominent fatty degeneration and necrosis were found and the normal architecture of the liver lobules was markedly destroyed, most of which were replaced by pseudolobules (Figure 1B, specimen from CW10A). Compared with immune induced group, fibrotic septum in CCL4 group was wide and compact.
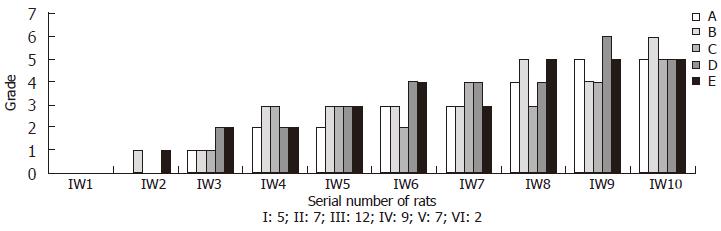
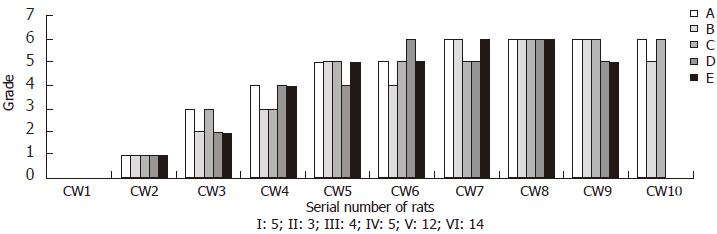
All rats in immune induced group survived the experiment. In tissue specimens of IW1A-E, only slight hepatocyte swelling could be observed, whereas no obvious damage presented. Histopathological stages of the five specimens were all determined to be stage 0. Among the specimens of IW2-5, obvious hyperplasia of connective tissues could be detected, but most of the histopathological stages were not above III. Among rats of immune induced group taken out after fifth week, with induction time extending, more livers were determined to be stage IV and V fibrosis. However, specimens determined to be stage VI were few (Only two fibrosis liver specimens of rats taken out at tenth week were determined to be stage VI) (Figure 2). Seven rats in CCL4 induced group died in the process of experiment (5 rats died before wk 5 and 2 died at wk 7), which livers were immediately excised and frozen at -70°C. Histopathological observation of the died rats showed severe liver damages existed in the liver such as inflammation, necrosis, excessive collagen deposition and inflammatory cells infiltration, etc. Obvious damage already existed in the liver tissue of the five rats of CW1, but no fiber hyperplasia presented. Histopathological stages were stage 0 according to the criteria described above. Specimens of CW2 presented obvious hepatocyte degeneration and necrosis, and vacuole degeneration and inflammatory cell infiltration also could be observed, but fiber hyperplasia was not predominant. The histopathological stages were determined to be stage I. From wk 3, normal architecture of the liver was rather lost, replaced with volumes of connective tissue. Hereafter, with the passage of time, fibrotic separations increased between the portal areas or between the portal area and the central vein, leading to the increasing formation of pseudolobules, and histopathologically stage IV or V was determined. In most of the rats taken out after wk 7, liver tissues were congested with pseudolobules and between the pseudolobules large numbers of collagens deposited, which were classified as classic stage VI (Figure 3).
In theory, reading (absorbency) of ELISA reader correlates with serum TIMP-1 level, which could properly reflect the quantity of serum TIMP-1[23,24].
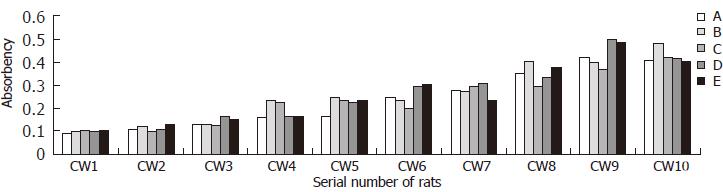
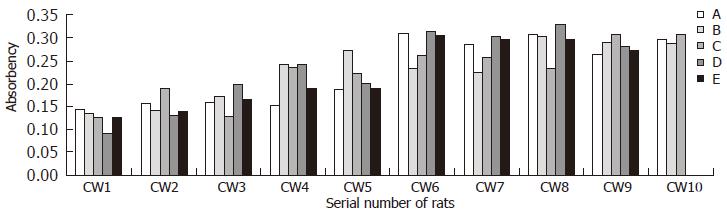
Results of serum TIMP-1 level of immune induced group and CCL4 induced group are presented in Figures 4 and 5 respectively. Data of control group accorded with normal distribution (mean = 0.105, 95% percentile = 0.137).
Positive correlation existed between serum TIMP-1 level of immune induced group and the histopathological stages of fibrosis liver of corresponding rats (Spearman rank-correlation test, rs = 0.812, P < 0.05), indicating serum TIMP-1 level could be considered as an index reflecting the degree of liver fibrosis.
No clear correlation between the serum TIMP-1 level of CCL4 induced group and the histopathological stages of corresponding rats (Spearman rank-correlation test, rs = 0.229, P > 0.05), indicating serum TIMP-1 level of CCL4 group could not reflect the degree of liver fibrosis. Even so, the serum TIMP-1 level of CCL4 group was obviously higher than that of control group (LSD-t, P < 0.001).
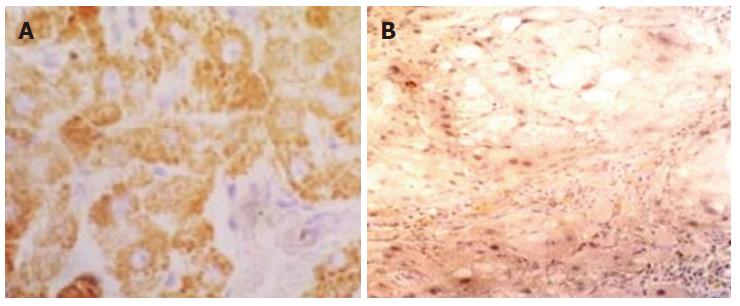
Seven specimens from immune group and CCL4 group respectively, which were all determined as stage V histopathologically (for CCL4 group, the seven specimens were randomly selected from the 12 stage V specimens), were used to compare the difference of TIMP-1 mRNA expression. Ten specimens of control group were detected at the same time. In the fibrosis liver of immune group, expression of TIMP-1 mRNA was detected in myofibroblasts, fibroblasts and vascular endothelial cells, especially predominant in the portal areas and fibrotic septum. The positive signals of TIMP-1 mRNA located in cytoplasm as brown particles, but not found in nucleus (Figure 6A). For CCL4 group, myofibroblasts, fibroblasts in the portal area and vascular endothelial cells expressed TIMP-1 mRNA. However, compared with immune group, the signals were weaker (Figure 6B).
Image pattern analysis of in situ hybridization revealed that among immune, CCL4 and control groups, expression of TIMP-1 mRNA of immune group was highest (t = 9.398, P < 0.05); while between CCL4 group and control group, expression of TIMP-1 mRNA of CCL4 group was obviously higher(t = 3.414, P < 0.05)(Table 2). The standard deviation of data of CCL4 group was distinctly higher than immune group (62.80 > 19.00), indicating greater variation existed in the specimens of CCL4 group even with the same histopathological stage.
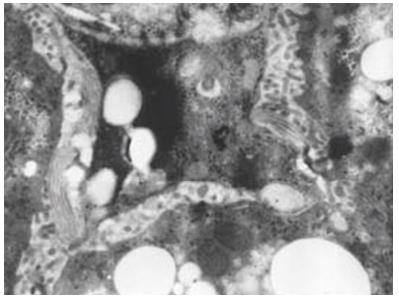
In liver fibrosis of immune group, large quantities of activated hepatic stellate cells (HSC) were detected. In the cytoplasm of activated HSCs, there were abundant rough endoplasmic reticulum, but fatty droplets were obviously decreased (Figure 7). Around the activated HSCs, in Disse space, between hepatocytes, and surrounding areas of bile duct, dense deposition of collagens could be found. Portal areas extended, where great number of myofibroblasts existed. In denatured hepatocytes, many swelling mitochondria and areas of fatty drops existed. For CCL4 group, severe collagen deposition could be observed, but with fewer activated HSCs.
Hepatic fibrosis is a consequence of different chronic liver diseases caused by hepatitis viruses, drugs, alcohol, parasite, and autoimmune mechanism. Nowadays there are several kinds of animal model of liver fibrosis for the study of the mechanisms of liver fibrosis, among which CCL4-induced rat liver fibrosis model and immune-induced rat liver fibrosis model were extensively investigated and applied[25]. Fibrosis caused by different pathogens has different pathogenesis processes. Therefore, not every fibrosis model is universally suitable. Which model should be applied in a given study is of great importance. But now, there is still no clear guideline for this.
Over the past 15 years, substantial progress has been made in understanding the role of TIMPs (TIMP-1 and TIMP-2) in the regulation of hepatic fibrosis. Hepatic fibrosis is a pathological process with the net deposition of ECM proteins[26]. The change of ECM is mainly regulated by MMPs. MMPs are a family of proteolytic enzymes that are capable of degrading the ECM and the activity of MMPs is tightly regulated by the amount of active protein and the concentration of TIMPs[27]. It has been identified that TIMP-1 plays a predominant role in regulating fibrosis by inhibiting the activity of MMP-1, MMP-3, MMP-9, and TIMP-1 is far stronger than TIMP-2[28]. So, TIMP-1 has been considered as an important factor in the progression of liver fibrosis and the expression level could be used as a new index of fibrosis severity.
In our previous studies[13,14], we found that the ex-pression of antigen and mRNA of TIMP-1 in liver tissue of CCL4-induced fibrosis rat was weaker compared with immune-induced fibrosis. Although higher than normal rat liver, the expression of TIMP-1 of CCL4-induced liver fibrosis was not consistent with the severity of hepatic fibrosis. In the current study, the fibrosis in CCL4-induced rat liver fibrosis model developed fast and almost all the histopathological grading of the rats which survived the experiment in this group belonged to grade V, and VI. Fibrous tissue in the fibrosis liver of this model was presented as wide and compact fibrotic septum, and most of the normal architecture of the liver lobules were destroyed and replaced by pseudolobules. But, in situ hybridization and immunohistochemical staining showed mRNAs and antigens of TIMP-1 expressed weakly, not proportional to the severity of the fibrosis. While, in immune-induced rat liver fibrosis model, even the fibrotic septum were small and sparse and histopathological grading was lower, the expression of mRNAs and antigens of TIMP-1 was higher compared with CCL4-induced rat liver fibrosis model. We used Spearman rank-correlation test to analyse the correlation between the serum levels of TIMP-1 and the histopathological stages of corresponding rat, and found that no clear correlation existed between the serum levels of TIMP-1 and the histopathological stages of corresponding CCL4-induced rat. While, serum levels of TIMP-1 of immune-induced fibrosis liver could reflect the severity of liver fibrosis. In the process of experiment, we failed to detect clear pathologic progression in the inducing process of CCL4, because the pathological changes developed very fast. We also analyzed the results of in situ hybridization of TIMP-1 mRNA in CCL4-induced fibrosis liver, which indicates that even with same histopathological stage, the TIMP-1 mRNA expression varied greatly, which was distinct from immune-induced rat liver fibrosis.
Currently studies on fibrosis are mostly focused on chronic hepatitis caused by hepatitis virus infection, which is a chronic progressive process. Our study revealed that the fibrosis induced by CCL4 progressed fast, and autopsies of the fibrosis livers presented hepatocyte vacuole degeneration and necrosis at the early period of CCL4 induction, indicating the subsequent ECM deposition might be the recovery process, during which connective tissue replaced the damaged architecture. The nosogenesis of CCL4 induced fibrosis may contribute to this change[29]. The hepatotoxicity of CCL4 results from its metabolites, the free radicals CCL3-, which could damage the hepatocyte by causing lipid peroxidation and by binding covalently to cell structures, leading to flaky hepatocyte vacuolar degeneration and necrosis. This is of obvious difference from the known fibrosis mechanism of viral hepatitis (HBV and HCV), which has been considered as a consequence of immunologic injury caused by the virus infection.
The fibrosis of immune-induced rat model is caused by type III allergy (immune complex type)[30]. The severity of the fibrosis is not so much high as CCL4-induced rat liver fibrosis, while the progression is similar to human viral hepatitis. Immune complex in serum exerts its hepatotoxicity through activating HSCs that can express TIMP-1, which enhances the deposition of ECM through inhibiting the activity of MMPs[31]. Under TEM, larger numbers of activated HSCs supported the above hypothesis.
In conclusion, the pathological changes of immune-induced rat liver fibrosis model is a gradual process and serum TIMP-1 level can reflect the severity of fibrosis. CCL4-induced rat liver fibrosis model develops fast in comparison with immune-induced rat liver fibrosis model and no significant correlation exists between the serum TIMP-1 level and the severity of fibrosis, both indicating that the model is unsuitable for experimental observation. Taken together of the difference of the two models, we think immune-induced rat liver fibrosis model is more suitable for the study of human chronic viral hepatitis.
We acknowledge the advice and help of Professo Meng-Dongr Li.
S- Editor Wang J L- Editor Zhu LH E- Editor Liu WF
| 1. | Abraham D, Ponticos M, Nagase H. Connective tissue remodeling: cross-talk between endothelins and matrix metalloproteinases. Curr Vasc Pharmacol. 2005;3:369-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Schuppan D, Ruehl M, Somasundaram R, Hahn EG. Matrix as a modulator of hepatic fibrogenesis. Semin Liver Dis. 2001;21:351-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 389] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 3. | Wang Y, Zhang JS, Huang GC, Cheng Q, Zhao ZH. Effects of adrenomedullin gene overexpression on biological behavior of hepatic stellate cells. World J Gastroenterol. 2005;11:3549-3553. [PubMed] |
| 4. | Parsons CJ, Bradford BU, Pan CQ, Cheung E, Schauer M, Knorr A, Krebs B, Kraft S, Zahn S, Brocks B. Antifibrotic effects of a tissue inhibitor of metalloproteinase-1 antibody on established liver fibrosis in rats. Hepatology. 2004;40:1106-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 158] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 5. | Shi GF, Li Q. Effects of oxymatrine on experimental hepatic fibrosis and its mechanism in vivo. World J Gastroenterol. 2005;11:268-271. [PubMed] |
| 6. | Murawaki Y, Ikuta Y, Idobe Y, Kitamura Y, Kawasaki H. Tissue inhibitor of metalloproteinase-1 in the liver of patients with chronic liver disease. J Hepatol. 1997;26:1213-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Murawaki Y, Ikuta Y, Idobe Y, Kawasaki H. Serum matrix metalloproteinase-1 in patients with chronic viral hepatitis. J Gastroenterol Hepatol. 1999;14:138-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Murawaki Y, Ikuta Y, Okamoto K, Koda M, Kawasaki H. Diagnostic value of serum markers of connective tissue turnover for predicting histological staging and grading in patients with chronic hepatitis C. J Gastroenterol. 2001;36:399-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Leroy V, Monier F, Bottari S, Trocme C, Sturm N, Hilleret MN, Morel F, Zarski JP. Circulating matrix metalloproteinases 1, 2, 9 and their inhibitors TIMP-1 and TIMP-2 as serum markers of liver fibrosis in patients with chronic hepatitis C: comparison with PIIINP and hyaluronic acid. Am J Gastroenterol. 2004;99:271-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 181] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 10. | Cai WM, Zhang BB, Weng HL, Hu ZR, Lv J, Zheng M, Liu RH. [The diagnostic value of eight serum indices for liver fibrosis]. Zhonghua Gan Zang Bing Za Zhi. 2004;12:219-222. [PubMed] |
| 11. | El-Gindy I, El Rahman AT, El-Alim MA, Zaki SS. Diagnostic potential of serum matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-1 as non-invasive markers of hepatic fibrosis in patients with HCV related chronic liver disease. Egypt J Immunol. 2003;10:27-35. [PubMed] |
| 12. | Afdhal N. Can serum markers be used to reliably detect liver fibrosis. Nat Clin Pract Gastroenterol Hepatol. 2005;2:132-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Nie QH, Cheng YQ, Xie YM, Zhou YX, Bai XG, Cao YZ. Methodologic research on TIMP-1, TIMP-2 detection as a new diagnostic index for hepatic fibrosis and its significance. World J Gastroenterol. 2002;8:282-287. [PubMed] |
| 14. | Nie QH, Duan GR, Luo XD, Xie YM, Luo H, Zhou YX, Pan BR. Expression of TIMP-1 and TIMP-2 in rats with hepatic fibrosis. World J Gastroenterol. 2004;10:86-90. [PubMed] |
| 15. | Nie Q, Zhou Y, Xie Y. [Expression and significance of tissue inhibitors of metallproteinases-1 and -2 in serum and liver tissue of patients with liver cirrhosis]. Zhonghua Yi Xue Za Zhi. 2001;81:805-807. [PubMed] |
| 16. | Wang BE, Wang ZF, Ying WY, Huang SF, Li JJ. The study on animal model of experimental liver fibrosis. Zhonghua Yixue Zazhi. 1989;69:503-505. |
| 17. | Maya-Mendoza A, Hernández-Muñoz R, Gariglio P, Aranda-Anzaldo A. Gene positional changes relative to the nuclear substructure during carbon tetrachloride-induced hepatic fibrosis in rats. J Cell Biochem. 2004;93:1084-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Wang BE, Wang HJ, Zhu JX, Iiu EY. Experimental and clinical study of the therapeutic effect of composite salviae multiorrhizae on liver fibrosis. Ganzangbing Zazhi. 1993;1:69-72. |
| 19. | Zhang BB, Cai WM, Weng HL, Hu ZR, Lu J, Zheng M, Liu RH. Diagnostic value of platelet derived growth factor-BB, transforming growth factor-beta1, matrix metalloproteinase-1, and tissue inhibitor of matrix metalloproteinase-1 in serum and peripheral blood mononuclear cells for hepatic fibrosis. World J Gastroenterol. 2003;9:2490-2496. [PubMed] |
| 20. | Jung K. Serum or plasma: what kind of blood sample should be used to measure circulating matrix metalloproteinases and their inhibitors. J Neuroimmunol. 2005;162:1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Tang X. [Serum TIMP-1 concentration in patients with chronic glomerulonephritis and the effect of losartan]. Di Yi Jun Yi Da Xue Xue Bao. 2003;23:966-969. [PubMed] |
| 22. | Wang H, Li Q, Shao L, Zhu C. Expression of matrix metalloproteinase-2, -9, -14, and tissue inhibitors of metalloproteinase-1, -2, -3 in the endometrium and placenta of rhesus monkey (Macaca mulatta) during early pregnancy. Biol Reprod. 2001;65:31-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Walsh KM, Timms P, Campbell S, MacSween RN, Morris AJ. Plasma levels of matrix metalloproteinase-2 (MMP-2) and tissue inhibitors of metalloproteinases -1 and -2 (TIMP-1 and TIMP-2) as noninvasive markers of liver disease in chronic hepatitis C: comparison using ROC analysis. Dig Dis Sci. 1999;44:624-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 102] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 24. | Kobayashi H, Li ZX, Yamataka A, Lane GJ, Miyano T. Clinical evaluation of serum levels of matrix metalloproteinases and tissue inhibitors of metalloproteinases as predictors of progressive fibrosis in postoperative biliary atresia patients. J Pediatr Surg. 2002;37:1030-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Sun MA, Wang BE, Annoni G, Degli Esposti S, Biempica L, Zern MA. Two rat models of hepatic fibrosis. A morphologic and molecular comparison. Lab Invest. 1990;63:467-475. [PubMed] |
| 26. | Muriel P, Moreno MG, Hernández Mdel C, Chávez E, Alcantar LK. Resolution of liver fibrosis in chronic CCl4 administration in the rat after discontinuation of treatment: effect of silymarin, silibinin, colchicine and trimethylcolchicinic acid. Basic Clin Pharmacol Toxicol. 2005;96:375-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Mott JD, Werb Z. Regulation of matrix biology by matrix metalloproteinases. Curr Opin Cell Biol. 2004;16:558-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 774] [Cited by in RCA: 799] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 28. | Knittel T, Mehde M, Grundmann A, Saile B, Scharf JG, Ramadori G. Expression of matrix metalloproteinases and their inhibitors during hepatic tissue repair in the rat. Histochem Cell Biol. 2000;113:443-453. [PubMed] |
| 29. | Weber LW, Boll M, Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit Rev Toxicol. 2003;33:105-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 1149] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 30. | Rosenberg WM. Rating fibrosis progression in chronic liver diseases. J Hepatol. 2003;38:357-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 31. | Xu L, Hui AY, Albanis E, Arthur MJ, O'Byrne SM, Blaner WS, Mukherjee P, Friedman SL, Eng FJ. Human hepatic stellate cell lines, LX-1 and LX-2: new tools for analysis of hepatic fibrosis. Gut. 2005;54:142-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 686] [Cited by in RCA: 845] [Article Influence: 42.3] [Reference Citation Analysis (0)] |