INTRODUCTION
γ-aminobutyric acid (GABA), a well-known inhibitory neurotransmitter, is also present in islet β-cells of the pancreas at a very high concentration comparable to that in the brain[1,2]. Glutamic acid decarboxylase (GAD) and GABA transaminase also co-exist in islet β-cells[3-5]. GABA has been known as a modulator of the endocrine pancreas. It has been reported that GABA and its agonist inhibit release of somatostatin[6] and glucagon[7] whereas it stimulates release of insulin[8]. The GABA concentration and the GAD activity are very high in insulinoma tissue[1] while the GAD level is very low in the pancreatic tissue with insulin-dependent diabetes mellitus[9].
In the pancreas, a substance secreted from islet cells reaches acinar cells at a very high concentration through the intra-pancreatic circulation, the islet-acinar portal system, and then gives a vast influence on exocrine function[10,11]. Thus, we hypothesized that GABA might also play a role in pancreatic exocrine function if it would be secreted from islet β-cells into the portal system. There are some reports suggesting that islet β-cells may secrete GABA and that GABA may affect pancreatic exocrine function. The pancreatic β-TC6 cells, a murine β-cell line developed from insulinoma, secrete GABA in response to glucose[12]. Cultured rat β-cells also secrete GABA into the medium in response to glutamine[13]. High affinity binding sites of GABA have been determined in pancreatic exocrine cells[14]. We have previously reported that exogenous GABA enhances CCK-stimulated exocrine secretion while it has not any influence on spontaneous or secretin-stimulated exocrine secretion of the isolated rat pancreas[15].
Thus, this study was aimed to investigate whether GABA in islet β-cells acts as a hormone to modify pancreatic exocrine secretion. Secretion of GABA from islet β-cells and effects of endogenous GABA on pancreatic exocrine secretion were determined in this study. Bicuculline was used to interfere with the GABA action by blocking GABAA receptors. Streptozotocin was employed to destroy islet β-cells containing GABA. The totally isolated, vascularly perfused pancreas model was used in this study to eliminate the possible influences of extrinsic nerves and hormones on exocrine secretion.
MATERIALS AND METHODS
Preparation of totally isolated and vascularly perfused pancreas
Male Sprague-Dawley rats, weighing 250-300 g, were anaesthetized with an intra-peritoneal injection of 25 g/L urethan (Sigma, St. Louis, MO) at a dose of 7 mL/kg of body mass after 24 h fasting with free access to water. Rats were sacrificed by an intravenous overdose of urethan after isolation of the pancreas. The isolated, vascularly perfused rat pancreas was prepared according to a method described previously[16,17]. In brief, the abdominal aorta was carefully dissected and cannulated with PE-50 tubing (Clay Adams, Parsippany, NJ) just above the celiac artery, and then tightly ligated below the superior mesenteric artery. The pancreatic duct was cannulated at the duodenal end with PE-10 tubing (Clay Adams). The portal vein was also cannulated with Tygon microbore tubing (Fisher Scientific, Pittsburgh, PA) to drain perfusate. The isolated pancreas was perfused with modified Krebs-Henseleit solution (pH 7.4, 305 mosmol/kg water) through the celiac and superior mesenteric arteries at a flow rate of 1.2 mL/min by using a multistaltic pump (Buchler, Kansas, MO). The perfusate contained 1 g/L bovine serum albumin (Sigma), 3 g/L Dextran T-70 (Sigma) and 18 mmol/L glucose (Sigma), and was continuously oxygenated with 95 mL/L O2 containing 5 mL/L CO2. The pancreas was isolated with the duodenum, but separated from other neighboring organs and tissues, and then placed in a temperature-controlled experimental chamber at 37 °C. The chamber was also continuously supplied with Krebs-Henseleit solution at a flow rate of 0.35 mL/min and also oxygenated. After an equilibration period of 30 min, pancreatic juice secreted in 15 min was collected throughout the whole period of the experiment. Portal effluent drained in 15 min was also collected in ice-chilled tubes, and then kept at -70 °C for GABA assay.
Effects of glutamine and GABA on CCK-induced exocrine secretion
Exocrine secretion of the isolated rat pancreas was stimulated by intra-arterial infusion of synthetic CCK-8 (Squibb Institute, Princeton, NJ) at a concentration of 10 pmol/L for 90 min. Glutamine (Sigma), a major precursor of GABA[14,18], was added to perfusate at a concentration of 1, 4, or 10 mmol/L from 30 min prior to the infusion of CCK until the end of the experiment. For mimicking the effects of glutamine, GABA (Sigma) at a concentration of 30 μmol/L was intra-arterially infused to the pancreas from 30 min before the CCK infusion until the end of the experiment. Bicuculline (Tocris, Baldwin, MO), a GABAA receptor antagonist[15,19], was intra-arterially infused at a concentration of 10 μmol/L from 30 min before the infusion of CCK until the end of the experiment. Effects of glutamine (4 mmol/L) on the CCK-stimulated pancreatic exocrine secretion were also observed after destruction of islet β-cells by streptozotocin (Sigma). Streptozotocin was intra-peritoneally injected at a single dose of 75 mg/kg 3 d before the experiment.
Effects of glutamine on GABA secretion
Glutamine (Sigma) was intra-arterially infused to the isolated pancreas at a concentration of 1, 4, or 10 mmol/L from 30 min prior to the infusion of CCK until the end of the experiment. The GABA concentration in portal effluent drained in 15 min was determined by an enzymatic recycling for NADPH as reported previously[1,14]. Briefly, 500 μL portal effluent of the isolated perfused rat pancreas was lyophilized. The lyophilized samples were dissolved in 80 μL GABA assay reagent (0.3 mol/L Tris-HCl buffer-pH 8.9, 10 002 nkat/L GABAse, 5 mmol/L α-ketoglutarate, 0.1 g/L mercaptoethanol, 0.5 mmol/L NADP+) and incubated for 30 min at 37 °C. At the end of the reaction, 20 μL of 1.5 mol/L NaOH was added and the incubation was continued for 20 min at 60 °C. A 20 μL aliquot was mixed with 250 μL enzyme recycling reagent (0.2 mol/L Tris-HCl buffer-pH 8.0, 5 mmol/L ketoglutarate, 1 mmol/L glucose-6-phosphate, 25 mmol/L ammonium acetate, 1 mmol/L ADP, 0.2 g/L BSA, 55,011 nkat/L glutamate dehydrogenase, 2.5 mmol/L glucose-6-phosphate dehydrogenase). The reaction was carried out at 37 °C for 1 h and stopped by heating at 100 °C for 7 min. A 90 μL aliquot was mixed with 250 μL assay reagent (0.1 mmol/L Tris-HCl buffer-pH 8.0, 1 mmol/L NADP+, 0.4 mmol/L EDTA, 366.7 nkat/L 6-phosphogluconate dehydrogenase) and incubated for 30 min at 25 °C. The final absorbance was recorded at 340 nmol/L. All chemicals were purchased from Sigma.
Pancreatic secretions of fluid and amylase
The volume flow of pancreatic juice was determined by measuring the length of pancreatic juice collected in microtube with a capacity of 3.8 μL/cm. α-amylase activity in pancreatic juice was determined by a method reported previously [20,21].
Statistical analysis
All results were illustrated as mean ± SE. The data were analyzed using the Student’s t test. The difference was considered significant when P < 0.05.
RESULTS
Effects of glutamine on CCK-stimulated exocrine secretion
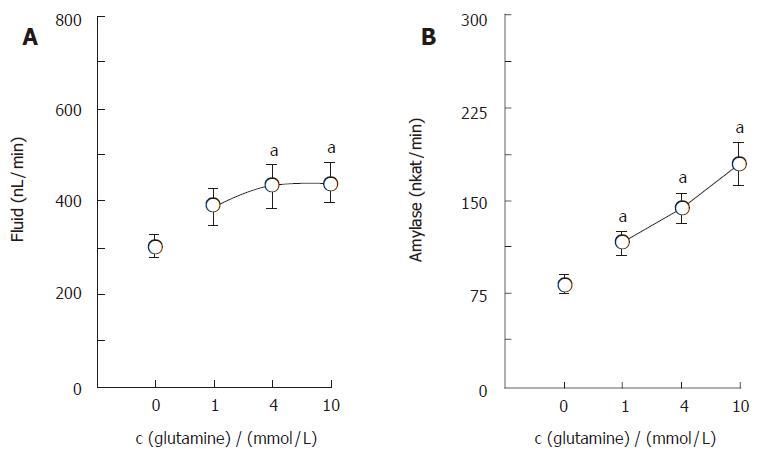
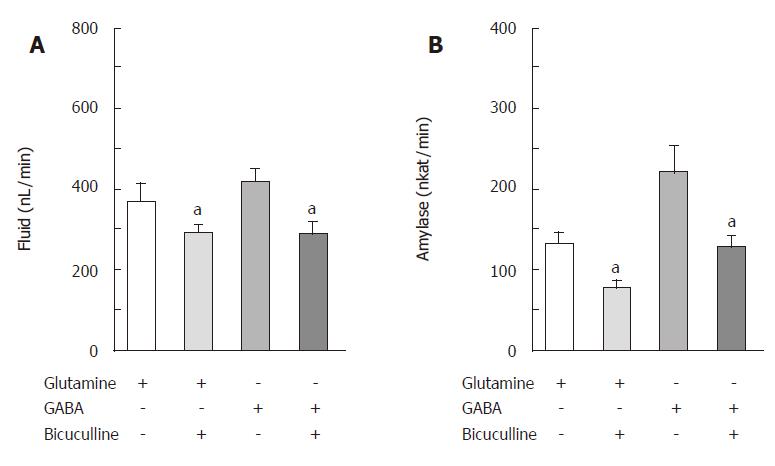
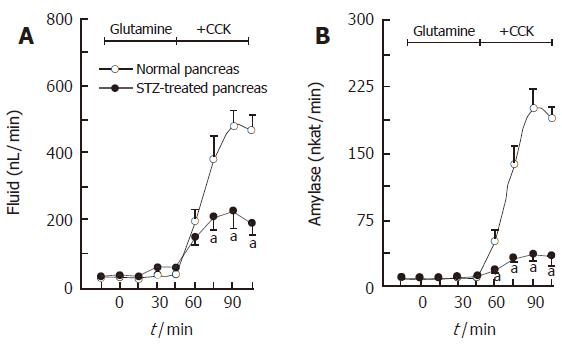
The isolated, perfused rat pancreas spontaneously secreted a minute amount of juice (34.8 ± 5.8 nL/min) and amylase activity (5.80 ± 1.86 nkat/min). CCK-8, given intra-arterially at a concentration of 10 pmol/L, significantly increased (P <0.01) the basal pancreatic secretions of fluid and amylase to 304.7 ± 23.5 nL/min and 82.83 ± 7.11 nkat/min, respectively. Figure 1 illustrates the effects of glutamine on the CCK-stimulated exocrine secretions of the isolated rat pancreas. Glutamine, added in the perfusate at concentrations of 1, 4 and 10 mmol/L, dose-dependently elevated the CCK-stimulated pancreatic secretions of fluid and amylase to 387.3 ± 39.0 nL/min and 115.71 ± 9.50 nkat/min, 432.3 ± 48.3 nL/min and 144.02 ± 11.88 nkat/min, 439.0 ± 45.3 nL/min and 179.18 ± 17.48 nkat/min, respectively. Figure 2 illustrates the effects of bicuculline, a GABAA receptor antagonist, on actions of glutamine and GABA in pancreatic exocrine secretions. Synthetic GABA, given intra-arterially at a concentration of 30 μmol/L, also increased (P < 0.001) the CCK-stimulated pancreatic secretions of fluid and amylase to 418.7 ± 31.8 nL/min and 221.82 ± 32.11 nkat/min, respectively. Bicuculline at a concentration of 10 μmol/L significantly reduced (P <0.001) the glutamine (4 mmol/L)- or GABA (30 μmol/L)-enhanced, CCK-stimulated pancreatic secretions of fluid and amylase to 289.3 ± 22.0 nL/min and 78.72 ± 7.00 nkat/min or 290.2 ± 27.7 nL/min and 130.00 ± 13.41 nkat/min, respectively. The enhancing effects of glutamine (4 mmol/L) on the CCK-stimulated secretions of fluid and amylase were greatly diminished in the pancreas isolated from the streptozotocin-treated rats (Figure 3).
Figure 1 Dose-dependent effects of glutamine on CCK-stimulated secretions of fluid (A) and amylase (B) in the isolated, perfused rat pancreas (mean ± SE, n = 7).
aP < 0.05 vs without glutamine.
Figure 2 Effects of bicuculline on glutamine- or GABA-enhanced, CCK-stimulated secretions of fluid (A) and amylase (B) in the isolated, perfused rat pancreas (mean ± SE, n = 7).
aP < 0.05 vs without bicuculline.
Figure 3 Effects of glutamine on CCK-stimulated secretions of fluid (A) and amylase (B) in the pancreas isolated from the streptozotocin (STZ)-treated rat (mean ± SE, n = 7).
aP < 0.05 vs STZ-treated pancreas.
Effects of glutamine on GABA secretion
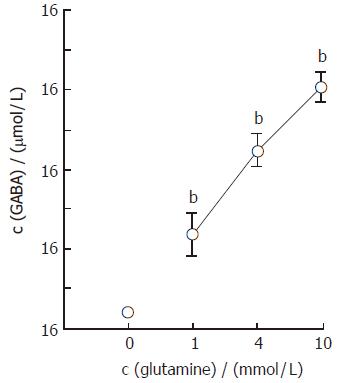
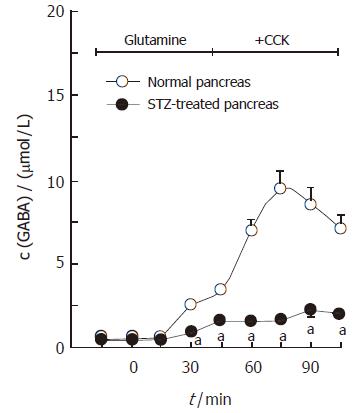
The mean basal concentration of GABA in portal effluent of the normal pancreas was 0.72 ± 0.08 μmol/L. Figure 4 demonstrates the dose-dependent effects of glutamine on GABA secretion in the isolated rat pancreas. When glutamine, at concentrations of 1, 4 and 10 mmol/L, was added to the perfusate containing 10 pmol/L CCK, the GABA concentrations in portal effluent increased from the basal level to the peak level of 4.72 ± 1.01 μmol/L, 8.94 ± 0.81 μmol/L and 12.11 ± 0.75 μmol/L, respectively. Figure 5 illustrates the effects of glutamine on GABA secretion in the pancreas isolated from streptozotocin-treated rats. The streptozotocin-treated pancreas spontaneously secreted GABA at a rate of 0.55 ± 0.04 μmol/L, which was significantly lower (P < 0.05) than that obtained in the normal pancreas. When glutamine, at 4 mmol/L, was added to the perfusate containing 10 pmol/L CCK, the GABA concentration was increased from the basal level to the peak level of 2.08 ± 0.28 μmol/L, which was also significantly lower (P < 0.001) than that observed in the normal pancreas.
Figure 4 Dose-dependent effects of glutamine on GABA secretion in the pancreas isolated from the normal rat (mean ± SE, n = 7).
bP < 0.01 vs without glutamine.
Figure 5 Effects of gluta-mine on GABA secretion in the pancreas isolated from the streptozotocin-treated rat (mean ± SE, n = 7).
aP < 0.05 vs normal pancreas.
DISCUSSION
The results of the present investigation clearly demonstrate that glutamine has an influence on exocrine secretion in the isolated, perfused rat pancreas. When glutamine was intra-arterially given at a concentration of 1, 4 or 10 mmol/L to the isolated pancreas, the CCK-stimulated pancreatic secretions of fluid and amylase were further elevated in a dose-dependent manner. Synthetic GABA, administered intra-arterially at a concentration of 30 μmol/L, also further increased the CCK-stimulated pancreatic secretions of fluid and amylase, as demonstrated previously[15]. Bicuculline, a GABAA receptor antagonist[19], effectively blocked the enhancing effects of glutamine or GABA on the CCK-stimulated pancreatic secretions. Because glutamine is a major precursor of GABA[18], the results strongly suggest that glutamine may enhance the CCK-stimulated pancreatic exocrine secretion by inducing secretion of GABA. So far, there is no direct evidence that GABA is secreted from the islet β-cells into the intra-pancreatic circulation although cultured β-cells secrete GABA into medium[14]. Thus, we examined whether GABA is secreted from the β-cells into the portal circulation of the pancreas in this study. When glutamine was added in the perfusate at concentrations of 1, 4 and 10 mmol/L, the GABA concentration in portal effluent was elevated dose-dependently. The effect of glutamine on the CCK-stimulated exocrine secretion was reduced in the pancreas isolated from rats treated with streptozotocin, a well-known β-cell toxin. Furthermore, not only at the basal state but also at the glutamine-infused state, the GABA concentration in portal effluent was much lower in the streptozotocin-treated pancreas than in the normal pancreas. The results firstly show that GABA is secreted by a secretagogue from islet β-cells into the intra-pancreatic circulation, which enhances the CCK-stimulated pancreatic exocrine secretion. There is good evidence that β-cells may secrete GABA. It has been reported that β-cells contain not only GABA but also GAD, a GABA synthesizing enzyme at high concentration[1,2,22]. β-cells include synaptic-like microvesicles that may be concerned with GABA secretion[22]. The pancreatic β-TC6 cells[13] or cultured rat β-cells[14] secrete GABA into the medium in response to glucose or glutamine. GABA may be also secreted from the GABAergic neurons in the pancreas because GABA-containing neuronal cell bodies locate at the periphery of islets, and numerous GABA-containing processes of the neurons extend into the exocrine pancreas[23]. Secretion of GABA from the GABA-containing neurons should be elucidated in future studies.
Endogenous GABA appears to exert the enhancing effects on the CCK-stimulated pancreatic exocrine secretion via GABAA receptors. In this study, the enhancing effects of glutamine on the CCK-stimulated pancreatic secretion were effectively blocked by bicuculline, a GABAA receptor antagonist[15,19]. Existence of GABAA receptors has been reported in pancreatic exocrine cells of neonatal rats[12] and in AR42J cells, a pancreatic cancer cell line[24]. Endogenous GABA may elevate the CCK-stimulated pancreatic exocrine secretion indirectly by modulating release of islet hormones. It has been reported that GABA or its agonist inhibits release of somatostatin[6,25], which inhibits CCK-stimulated pancreatic exocrine secretion[26,27]. We have already reported that a somatostatin antagonist further elevates the enhancing effects of GABA on CCK-stimulated pancreatic exocrine secretion[15]. It has been also documented that GABA stimulates release of insulin[8], which increases CCK-stimulated pancreatic exocrine secretion[28,29]. In addition, the GAD activity in the pancreatic islet is very low in mice suffering from insulin-dependent diabetes mellitus[9], in which pancreatic exocrine secretion is reduced[30]. Endogenous GABA may also enhance the intrinsic neuronal action on pancreatic exocrine secretion. Our previous studies showed that exogenous GABA further elevates pancreatic exocrine secretion evoked by electrical field stimulation as well as neurotransmitters such as gastrin-releasing peptide[27] and acetylcholine[32] in rat pancreas. Recently, GABA-sensitive neurons have been electrophysiologically observed in cat pancreas[33]. Although GABA could depolarize all ganglial cells recorded in the study through GABAA receptor, only approximately 10% of neurons among them could generate action potential. Thus, it is unclear at present whether the GABA-induced neuronal activation results in modification of pancreatic exocrine secretion.
In summary, glutamine could further elevate CCK-stimulated pancreatic exocrine secretion of the isolated rat pancreas dose-dependently, which could be blocked by bicuculline. Glutamine could increase the GABA concentration in portal effluent of the isolated rat pancreas dose-dependently. The effects of glutamine on the CCK-stimulated pancreatic exocrine secretion as well as the GABA secretion were markedly reduced in the pancreas isolated from the streptozotocin-treated rat. Therefore, it is concluded that GABA can be secreted from islet β-cells into the islet-acinar portal system after administration of glutamine, which could enhance CCK-stimulated pancreatic exocrine secretion via GABAA receptors. The results strongly indicate that GABA in islet β-cells may be an islet hormone affecting exocrine secretion of the pancreas in rats.