Published online May 14, 2006. doi: 10.3748/wjg.v12.i18.2908
Revised: September 28, 2005
Accepted: November 18, 2005
Published online: May 14, 2006
AIM: To investigate the effects of hyperlipidemia on acute pancreatitis (AP) and the possible mechanisms.
METHODS: Rat models of hyperlipidemia and AP were established by Triton WR1339 and cerulein respectively. Human albumin was used to treat AP complicated by hyperlipidemia. In each group, we compared the histological score, volume of ascites, ratio of pancreatic wet/dry weight, serum amylase (AMY) and pancreatic acinar cell apoptosis. The level of protein kinase C (PKC) membrane translocation in pancreatic tissue was detected by Western blot.
RESULTS: In the hyperlipidemia model established by Triton WR1339, triglyceride (TG) increased remarkably and reached its peak 6 h after injection, and most rats developed mild acute pancreatitis. Histological score, volume of ascites, ratio of wet/dry weight and serum AMY in AP animals with hyperlipidemia were obviously higher than those in AP animals (P < 0.05) and decreased after albumin therapy but not significantly (P > 0.05). Apoptotic cells detected by terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) increased in AP animals with hyperlipidemia and did not change distinctly after albumin therapy. PKC membrane translocation level increased in AP animals with hyperlipidemia and decreased remarkably after albumin therapy (P < 0.05).
CONCLUSION: Hyperlipidemia may induce AP or intensify pancreatic injury. Albumin therapy can not alleviate pancreatic lesion effectively. PKC activation may be one mechanism by which AP is intensified by hyperlipidemia.
- Citation: Wang YJ, Sun JB, Li F, Zhang SW. Hyperlipidemia intensifies cerulein-induced acute pancreatitis associated with activation of protein kinase C in rats. World J Gastroenterol 2006; 12(18): 2908-2913
- URL: https://www.wjgnet.com/1007-9327/full/v12/i18/2908.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i18.2908
Acute pancreatitis (AP) is a common disease that normally runs a benign course in the majority of patients. However in up to 20% of individuals the disease is severe and may be associated with a mortality of about 20%[1,2]. Since Speck in 1865[3] noted the association between hyperlipidemia and AP, their relationship has been studied by many scholars. Currently, hyperlipidemia is another main cause of AP besides cholelithiasis and alcohol abuse[4]. Serum triglyceride (TG) levels higher than 1000 mg/dl seem to be a serious factor for developing AP, particularly in patients suffering from familial hyperlipidemia (types 1, 4 or 5 according to Fredrickson’s classification). AP occurs with an incidence of up to 21%[5,6]. Hydrolysis of TG by pancreatic lipase with the local release of large quantities of free fatty acids (FFAs) has been proposed as the pathogenic mechanisms. Hyperlipidemia is a common cause of AP. Moreover, lipid levels increase above normal in up to 50% of patients with AP of any cause[7].
Several studies in humans and animals have been performed to evaluate hyperlipidemia as a risk factor for developing AP. However, there is no ideal animal model of hyperlipidemic AP. Information about the influence of hyperlipidemia on the course of AP is relatively rare. The majority of investigators considered that hyperlipidemia can intensify the pancreatic lesion and systemic inflammatory response. Saharia et al[8] perfused the pancreas preparations with TG and FFAs (oleic acid) and found that they can lead to edema of the isolated pancreas, weight gain, and elevation of serum AMY. The injury caused by FFAs is similar to that seen with TG infusion, but it occurs more rapidly. These studies add support to the concept that hypertriglyceridemia can initiate pancreatic injury and the release of FFAs may be the mechanism. Kimuru et al[9] initiated an AP model with cerulein, retrograde duct injection of sodium taurocholate, bile/pancreatic duct respectively and found that the isolate pancreatic damage is increased by TG perfusion. However, some reports suggest that hyperlipidemia could not aggravate the course of AP. Paye et al[10] prepared an AP model with cerulein and injected TG simultaneously, and found that histological alterations, pancreatic enzyme level and ascites in these models do not differ from those in AP patients without TG injection. To approve this result further, the authors collected very low density lipoprotein (VLDL) from AP patients and found that the VLDL intravenous infusion for hypertriglyceridemia does not alter the course of AP[11]. Therefore the role of hyperlipidemia in acute pancreatitis seems questionable.
The mechanism by which hyperlipidemia leads to AP is still unclear. A well-accepted mechanism proposed by Havel et al[12] is that unbound FFAs are toxic and could produce acinar cells or capillary injury. Increased concentration of chylomicrons in pancreatic capillaries causes capillary plugging and leads to ischemia and acidosis. FFAs can induce activation of trypsinogen and initiate AP. Recently, it was reported that FFAs are toxic to pancreatic β-cells leading to diabetes and insulin resistance syndrome. The toxicity is associated with abnormalities in the insulin signaling cascade and may be mediated by FFA activation of protein kinase C (PKC)[13]. Also, there is abundant PKC in pancreatic acinar cells[14]. Other similar mechanisms of FFAs may damage acinar cells.
The aim of the present work was to determine whether endogenous hyperlipidemia aggravates in vivo cerulein-induced edematous AP and activates PKC in pancreas of rats.
Male Sprague-Dawley rats, weighing 250-270 g were used. Experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The rats were maintained at 23 °C in a 12 h light dark cycle, with free access to water and standard rat chow. The rats were fasted for 12 h prior to the experiment, but with free access to water. They were anesthetized with chloral hydrate (300 mg/kg intra-peritoneal injection). Body temperature was monitored and maintained at 37.5 ± 1 °C using a heating device.
Hyperlipidemia was induced by intravenous injection of 10% Triton WR1339 solution (TyloxAPol, Sigma, USA) through the left external iliac vein. Triton WR1339, a non-ionic surface active detergent leads to an increase of endogenous TG and total cholesterol (TCH) blood concentrations[15,16]. We determined the required amounts of Triton WR1339 with two doses of 100 mg/kg and 400 mg/kg body weight respectively. A volume of 400 mg/kg Triton WR1339 solution could induce maximal hyperlipidemia. The non-hyperlipidemic animals received an injection of physiological saline solution intravenously instead of Triton WR1339.
AP was induced by 4 h abdominal subcutaneous injection of cerulein (Sigma, American, 20 μg/kg in 0.5 mL of saline vehicle) as previously described[17].
To explore the influence of hyperlipidemia on acute experimental pancreatitis, we used five different groups of animals with 12 rats in each group. The animals of the first group [C(sal)] received 1.0 mL saline (sal) solution, and the second group [C(tri)] received 1.0 mL of 10% Triton WR1339 (tri) intravenously. In these two groups [C(sal) and C(tri)] no pancreatitis was induced. AP was induced in the third group (AP) and the fourth group (HAP) as described above. The animals of group HAP received an injection of 1.0 mL of Triton WR1339 intravenously 6 h before pancreatitis was induced with cerulein infusion. Since albumin could effectively combine FFAs in circulation to prevent cellular damage[18], in the fifth treatment group (THAP), the rats received an injection of 1.25 g/kg albumin intravenously after pancreatitis was induced.
Blood samples were taken from all animals before and 3, 6, 9 h after induction of hyperlipidemia or AP with hyperlipidemia. The samples were used to measure TG, TCH, AMY activity.
Three and six hours after induction of AP, the rats were sacrificed by aortic puncture and blood samples taken for plasma biochemical measurements. A complete macroscopic examination of the peritoneal cavity and ascites was immediately performed, and the pancreas was removed using a standardized monobloc surgical technique. The pancreas was divided into three parts. The pancreatic head was fixed in Bouin’s solution, embedded in paraffin, sliced, and stained with hematoxylin and eosin. The pancreas body was put into liquid nitrogen for PKC analysis. To better estimate the amount of edema, the remaining pancreatic tissue was weighed and dessicated for 24 h at 173 °C to measure its dry weight. We used the wet to dry ratio to quantify edema formation.
To quantify the histological changes, two pathologists judged the tissue samples using a point score as previously described[19,20]. Plasma AMY concentrations were determined after plasma dilution (1/20) by the colorimetric assay AMYL (Boehringer Mannhein, Germany). Plasma TG was measured using the colorimetric assay TG GPOPAP (Boehringer).
Recent studies indicate that mild acute pancreatitis is related to the extent of acinar cell apoptosis[21-23]. A pancreatic tissue section was prepared and assayed with the in situ cell death detection kit, POD (Boehringer Mannheim, Germany) following the manufacturer’s instructions. Slides were counterstained with hematoxylin and viewed under a light microscope.
PKC activation was determined in both cytosolic and particulate fractions as previously described[24]. Frozen pancreatic tissue(50 mg) was rapidly thawed and homogenized at 4 °C in lysis buffer A (mM: Tris-HCl 50; pH 7.4; NaCl 150; EDTA 2; EGTA 2; DTT 2; Na pyrophosphate 5; KF 50; PMSF 1; 1μg/mL: Aprotinin, Leupeptin,Pepstatin A). Homogenates were centrifuged at 100 000 r/min for 30 min at 4 °C in an Optima TLX ultracentrifuge to yield cytosol. The pellet fractions were resuspended in 100 μL of buffer A containing 0.5% Nonidet P-40, sonicated and centrifuged again. The resulting supernatants taken as the particulate fractions with the cytosolic fractions were assayed for PKC immunoreactivities. Throughout the study, protein concentrations were determined by the Lowry’s method with bovine serum albumin as standard. Aliquots of the cell lysates containing 100 g of total protein were resolved in 10% SDS-PAGE and transferred to PVDF membranes (Amersham Biosciences, Inc.). The membranes were blocked in TBS-Triton-X 100 (0.2% v/v, pH 7.4) containing 5% milk for 2 h at room temperature followed by incubation with PKC antibody at the dilution described by manufacturer’s instructions at 4 °C overnight. The membranes were incubated with anti-goat horseradish peroxidase complex (diluted 1:2000) for 2 h at room temperature. The proteins on the membranes were exposed to X-ray films at multiple time points and visualized by ECL detection system (Amersham Biosciences, Inc.).
Data were expressed as mean ± SD and analyzed using Student’s t test or Student’s Newman Keuls-test by SPSS 11.0 software. Quantitative analysis for immunoblot was done by scanning the x-ray film and determined using the Fotodyne Gel-Pro analyzer program.
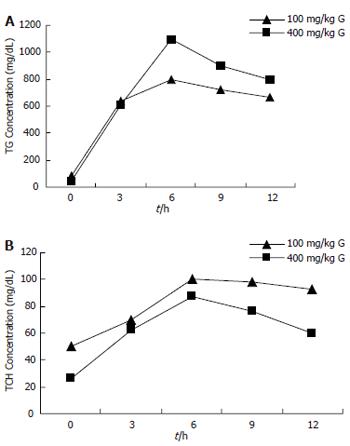
The animals receiving 100 mg/kg and 400 mg/kg Triton WR1339 had high TG and TCH. The serum TG increased most obviously and reached its peak 6 h after Triton WR1339 infusion with induction of chylomicronemia (Figure 1). The TG value increased 10-fold and 20-fold after 100 mg/kg and 400 mg/kg Triton WR 1339 infusion respectively, whereas the serum TCH increased only 2-fold and 3-fold (Figure 2). The serum AMY activities did not significantly increase after induction of hyperlipidemia (P > 0.05). The animals did not demonstrate ascites and pancreatic edema in macroscopy. Nine rats (9/12) showed mild pancreatic edema, whereas no histological changes occurred in the saline-treated animals (group C(sal)).
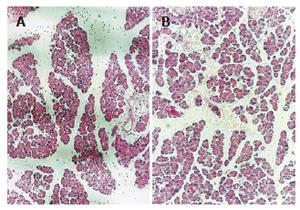
The pancreatic changes included interstitial edema, increased interlobular interstice and infiltration of some inflammatory cells in the animals 3 h after cerulein infusion. The pancreas of AP animals 6 h after cerulein injection had serious edema with tensioned capsules and separated small lobuli. Wide lobular and acinar interstices, anamorphic acinar structure, intracytoplasmic vacuolization and infiltrated inflammatory cells were observed (Figure 3). After 3 h, the pancreatic damage was intensified in the animals of group HAP,and some apoptotic bodies (inclusion) and more inflammatory cells appeared. There were scattered erythrocytes and inflammatory cells, distended small veins in the pancreas 6 h after HAP model was induced (Figure 3). There were no pathological changes after albumin therapy.
The animals receiving cerulein (group C(tri)) had significantly (P < 0.05) higher pancreatic histological score, volume of ascites and pancreatic wet/dry ratio than the control group. The pancreatic histological score, volume of ascites and pancreatic wet/dry ratio were significantly higher (P < 0.05) in the AP animals with hyperlipidemia (group HAP) than in the animals receiving only cerulein infusion (group C(tri)). After albumin treatment (group THAP) these parameters decreased slightly but were higher in the 6 h group than in the 3 h group (P > 0.05). These results suggested that hyperlipidemia could aggravate pancreatic edema of the AP animals and albumin therapy was not obviously effective (Table 1).
| Group | Time | Histological score | Ascites(ml) | Wet/dry |
| C(sal) | 3 h | 0.33 ± 0.52 | 0 | 1.35 ± 0.09 |
| 6 h | 0.33 ± 0.52 | 0 | 1.35 ± 0.09 | |
| AP | 3 h | 3.50 ± 1.38a | 0.87 ± 0.58 | 1.67 ± 0.05a |
| 6 h | 5.33 ± 1.03a | 1.58 ± 0.92 | 1.76 ± 0.14a | |
| HAP | 3 h | 5.17 ± 1.17c | 2.08 ± 0.58c | 1.95 ± 0.44 |
| 6 h | 6.83 ± 1.16c | 4.12 ± 1.58c | 2.15 ±0.39 | |
| THAP | 3 h | 4.83 ± 1.16 | 1.87 ± 0.93 | 1.72 ± 0.14 |
| 6 h | 6.67 ± 1.21 | 3.68 ± 1.42 | 1.88 ± 0.33 |
The serum TG and TCH concentration increased 1-2 folds in the cerulein-induced AP animals. There were no statistical differences between group AP and group C(sal) (P > 0.05). The hyperlipidemic AP animals had significantly higher serum TG and TCH values than the rats receiving Triton WR1339 (group C(tri)) injection. The serum TG and TCH concentration in the animals with albumin therapy (group THAP) decreased slightly. The AMY activities in the hyperlipidemic group C(tri) remained unchanged throughout the investigation, and increased obviously (P < 0.05) in the AP group. In comparison with the AP group, the AMY values of group HAP and THAP were significantly higher (P < 0.05). This result demonstrated that AP could induce mild hyperlipidemia. Albumin could not decrease the circulating lipid and AMY concentration (Table 2).
| Group | Time | TG | TCH | AMY |
| C(sal) | - | 47.00 ± 23.38 | 26.25 ± 2.36 | 1658.50 ± 335.21 |
| AP | 3 h | 63.66 ± 11.52 | 61.00 ± 6.00 | 5128.66 ± 259.65 |
| 6 h | 72.66 ± 22.65 | 67.00 ± 8.71 | 6641.00 ± 213.75 | |
| C(tri) | 3 h | 899.51 ± 240.52 | 76.03 ± 16.14 | 1652.55 ± 300.46 |
| 6 h | 797.07 ± 356.18 | 60.51 ± 33.49 | 1253.72 ± 254.80 | |
| HAP | 3 h | 1604.16 ± 248.10a | 264.33 ± 50.33a | 18390.00 ± 7158.77a |
| 6 h | 1506.66 ± 327.72c | 230.33 ± 31.87c | 11611.66 ± 4513.67c | |
| THAP | 3 h | 1396.83 ± 658.65 | 221.00 ± 60.46 | 20473.33 ± 12005.46 |
| 6 h | 1167.33 ± 228.28 | 211.00 ± 14.02 | 16524.39 ± 5273.52 |
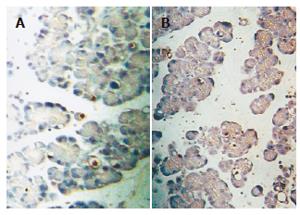
It was reported that the extent of acinar cell apoptosis is related to the severity of mild AP[22]. Careful biochemical and morphological examination of experimental models of AP has shown that severe AP is associated with necrosis, whereas mild acute pancreatitis is associated with apoptotic cell death[20,21]. In our study, the TUNEL methods was used for in situ apoptotic acinar cell identification. The apoptotic cells were not visible in the pancreas of control group, but a few apoptotic cells (brown) were visible in cerulein-induced AP animals. A subpopulation of apoptotic cells scattered throughout the tissue section was stained brown Figure 4, but the apoptotic cells did not markedly decrease after albumin therapy.
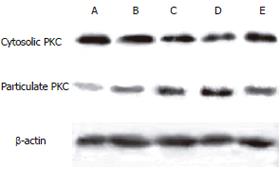
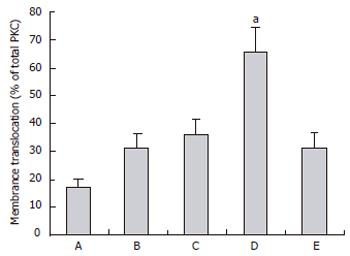
To confirm the activation of PKC, we examined the translocation of the enzyme from the cytosol to the particulate membrane by Western blot. The cytosolic PKC gradually decreased in the control group C(sal), hyperlipidemic group C(tri), cerulein-induced AP group and HAP group. After albumin treatment the cytosolic PKC increased remarkably in group THAP. However, the particulate PKC gradually increased in the above four groups and decreased after albumin therapy (Figure 5). The degree of PKC translocation was evaluated by the proportion of particulate PKC in the total PKC as previously described[25]. The translocation rate of PKC was significantly higher (P < 0.05) in hyperlipidemic AP animals (group HAP) than in non-hyperlipidemic AP rats (group AP) (Figure 6). In the hyperlipidemic AP group, the translocation rate of PKC was significantly decreased (P < 0.01) after albumin treatment. This result implied that hyperlipidemia could induce pancreatic PKC activation in normal and AP rats, which was consistent with the pancreatic injury. Albumin could reduce hyperlipidemia-induced PKC activation.
Hypertriglyceridemia is an etiological factor in patients with AP while TG level exceeding 1000 mg/dL or lactescent serum is a necessary condition for hyperlipidemic AP diagnosis[26]. Triton WR1339 could increase serum TG and is an ideal drug to establish hyperlipidemic animal model with TG elevation.
The rats without pancreatitis showed no clinical differences after Triton WR1339 infusion compared with saline infusion. Macroscopically, the pancreas of the hyperlipidemic and normal animals showed no difference. Microscopically, the animals treated with Triton WR1339 showed smooth edema, whereas no changes occurred in the non-hyperlipidemic animals, suggesting that severe hyperlipidemia may cause mild AP. The serum AMY activities were also slightly increased after hyperlipidemia induction, but there was no significant difference (P > 0.05). To investigate the effect of hyperlipidemia on AP, we established the AP model when the endogenous hyperlipidemia induced by Triton WR1339 reached its maximal value. Theoretically, significant influence of hyperlipidemia on AP would appear. We produced the hyperlipidemic AP animal model by injecting cerulein 6 h after Triton WR1339 infusion. It was reported that albumin could effectively alleviate the toxicity of TG to pancreatic cells[18]. A medium dose of albumin was injected through external iliac vein in the hyperlipidemia AP animals to observe the changes of pancreatic damage. As a result, hyperlipidemia aggravated pancreatic edema, volume of ascites and pancreatic cell apoptosis. The serum lipid in the hyperlipidemic AP animals increased compared with that in the hyperlipidemic animals, suggesting that hyperlipidemia may aggravate the course of AP while AP may exacerbate hyperlipidemia. There is a vicious circle between hyperlipidemia and AP. Accordingly, it is very important to decrease circulating lipid in clinical patients with AP. In our studies, the pancreatic damage aggravated by hyperlipidemia was not prevented by simultaneous perfusion of albumin, which might be due to the fact that the concentration of albumin in the inflamed pancreas may be too low or there may be other mechanisms of pancreatic injury. It was reported that FFAs could inhibit division and proliferation of pancreatic endocrine cells by activating PKC and induce apoptosis of pancreatic β-cells. As a result insulin secretion is decreased and diabetes occurs[27]. There is abundant PKC in pancreatic acinar cells[14]. Our study showed that FFAs could cause injury of pancreatic exocrine cells. PKC activity of pancreas increased in the hyperlipidemia and AP animals. The PKC activity changes were consistent with the degree of pancreatic injury, suggesting that PKC may participate in the course of hyperlipidemia inducing or aggravating pancreatic injury of AP. The PKC activity in hyperlipidemic AP animals was decreased significantly (P < 0.01) after albumin therapy, which implies that albumin may reduce the PKC activation through binding to FFAs. Although PKC activation decreased obviously (P < 0.01) after albumin therapy, the pancreatic injury was slightly relieved (P > 0.05), suggesting that PKC activation may be an important aspect of hyperlipidemia aggravating AP, and there may be other mechanisms of hyperlipidemia-induced AP.
S- Editor Guo SY L- Editor Wang XL E- Editor Bai SH
| 1. | Toouli J, Brooke-Smith M, Bassi C, Carr-Locke D, Telford J, Freeny P, Imrie C, Tandon R. Guidelines for the management of acute pancreatitis. J Gastroenterol Hepatol. 2002;17 Suppl:S15-S39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 265] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 2. | Zhu AJ, Shi JS, Sun XJ. Organ failure associated with severe acute pancreatitis. World J Gastroenterol. 2003;9:2570-2573. [PubMed] |
| 3. | Speck L. Fall von lipamia. Arch Verin Wissenschaftl Heilkunde 1865; 1: 232. Quoted in Thannhauser SJ, ed. Lipidoses, diseases of the intracellular lipid metabolism. 3rd ed. New York: Grune & Stratton 1958; 307. |
| 4. | Mao EQ, Tang YQ, Zhang SD. Formalized therapeutic guideline for hyperlipidemic severe acute pancreatitis. World J Gastroenterol. 2003;9:2622-2626. [PubMed] |
| 5. | Farmer RG, Winkelman EI, Brown HB, Lewis LA. Hyperlipoproteinemia and pancreatitis. Am J Med. 1973;54:161-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 60] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Cameron JL, Capuzzi DM, Zuidema GD, Margolis S. Acute pancreatitis with hyperlipemia: the incidence of lipid abnormalities in acute pancreatitis. Ann Surg. 1973;177:483-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 118] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Yadav D, Pitchumoni CS. Issues in hyperlipidemic pancreatitis. J Clin Gastroenterol. 2003;36:54-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 273] [Article Influence: 12.4] [Reference Citation Analysis (2)] |
| 8. | Saharia P, Margolis S, Zuidema GD, Cameron JL. Acute pancreatitis with hyperlipemia: studies with an isolated perfused canine pancreas. Surgery. 1977;82:60-67. [PubMed] |
| 9. | Kimura W, Mössner J. Role of hypertriglyceridemia in the pathogenesis of experimental acute pancreatitis in rats. Int J Pancreatol. 1996;20:177-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 106] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Paye F, Chariot J, Molas G, Benessiano J, Rozé C. Nonesterified fatty acids in acute cerulein-induced pancreatitis in the rat. Are they really deleterious in vivo. Dig Dis Sci. 1995;40:540-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Paye F, Chariot J, Molas G, Benessiano J, Rozé C. Release of nonesterified fatty acids during cerulein-induced pancreatitis in rats. Dig Dis Sci. 1996;41:1959-1965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Havel RJ. Pathogenesis, differentiation and management of hypertriglyceridemia. Adv Intern Med. 1969;15:117-154. [PubMed] |
| 13. | Haber EP, Ximenes HM, Procópio J, Carvalho CR, Curi R, Carpinelli AR. Pleiotropic effects of fatty acids on pancreatic beta-cells. J Cell Physiol. 2003;194:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 120] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Kim MJ, Lee YS, Lee KH, Min DS, Yoon SH, Hahn SJ, Kim MS, Jo YH. Site-specific localization of protein kinase C isoforms in rat pancreas. Pancreatology. 2001;1:36-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Takahashi Y, Inaba N, Kuwahara S, Kuki W. Effects of gamma-terpinene on lipid concentrations in serum using Triton WR1339-treated rats. Biosci Biotechnol Biochem. 2003;67:2448-2450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Hofbauer B, Friess H, Weber A, Baczako K, Kisling P, Schilling M, Uhl W, Dervenis C, Büchler MW. Hyperlipaemia intensifies the course of acute oedematous and acute necrotising pancreatitis in the rat. Gut. 1996;38:753-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Tani S, Otsuki M, Itoh H, Fujii M, Nakamura T, Oka T, Baba S. Histologic and biochemical alterations in experimental acute pancreatitis induced by supramaximal caerulein stimulation. Int J Pancreatol. 1987;2:337-348. [PubMed] |
| 18. | Kimura W, Meyer F, Hess D, Kirchner T, Fischbach W, Mössner J. Comparison of different treatment modalities in experimental pancreatitis in rats. Gastroenterology. 1992;103:1916-1924. [PubMed] |
| 19. | Schmidt J, Lewandrowsi K, Warshaw AL, Compton CC, Rattner DW. Morphometric characteristics and homogeneity of a new model of acute pancreatitis in the rat. Int J Pancreatol. 1992;12:41-51. [PubMed] |
| 20. | Ding SP, Li JC, Jin C. A mouse model of severe acute pancreatitis induced with caerulein and lipopolysaccharide. World J Gastroenterol. 2003;9:584-589. [PubMed] |
| 21. | Gukovskaya AS, Perkins P, Zaninovic V, Sandoval D, Rutherford R, Fitzsimmons T, Pandol SJ, Poucell-Hatton S. Mechanisms of cell death after pancreatic duct obstruction in the opossum and the rat. Gastroenterology. 1996;110:875-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 110] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Kaiser AM, Saluja AK, Sengupta A, Saluja M, Steer ML. Relationship between severity, necrosis, and apoptosis in five models of experimental acute pancreatitis. Am J Physiol. 1995;269:C1295-C1304. [PubMed] |
| 23. | Bhatia M. Apoptosis of pancreatic acinar cells in acute pancreatitis: is it good or bad. J Cell Mol Med. 2004;8:402-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 72] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Wu J, Li J, Huang KP, Huang FL. Attenuation of protein kinase C and cAMP-dependent protein kinase signal transduction in the neurogranin knockout mouse. J Biol Chem. 2002;277:19498-19505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Cui XY, Li JF, Han S, Zu PY. [Hypoxic preconditioning increases cPKCgamma membrane translocation in murine brain]. Shengli Xuebao. 2004;56:461-465. [PubMed] |
| 26. | Athyros VG, Giouleme OI, Nikolaidis NL, Vasiliadis TV, Bouloukos VI, Kontopoulos AG, Eugenidis NP. Long-term follow-up of patients with acute hypertriglyceridemia-induced pancreatitis. J Clin Gastroenterol. 2002;34:472-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 128] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 27. | Rakatzi I, Mueller H, Ritzeler O, Tennagels N, Eckel J. Adiponectin counteracts cytokine- and fatty acid-induced apoptosis in the pancreatic beta-cell line INS-1. Diabetologia. 2004;47:249-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 147] [Article Influence: 7.0] [Reference Citation Analysis (0)] |