Published online May 7, 2006. doi: 10.3748/wjg.v12.i17.2785
Revised: August 12, 2005
Accepted: December 12, 2005
Published online: May 7, 2006
AIM: To generate a SV40Tag transgenic tumor animal model and to study the mechanism underlying tumorigenesis.
METHODS: A mammary gland expression vector containing SV40Tag DNA was generated. Transgene fragments were microinjeted into fertilized eggs of FVB mice. The genetically manipulated embryos were transferred into the oviducts of pseudo-pregnant female mice. PCR and Northern blot analysis were used for genotype analysis of F1 and F2 mice. Transgene expression was detected by RT-PCR and immunohistochemistry.
RESULTS: SV40Tag gene was detected in two lines of transgenic mice. One of them delivered the transgene to F1 and a tumor was found in the pancreas of these mice. RT-PCR and immunohistochemistry showed that SV40Tag gene was expressed in the tumor. Pathological characterization of the transgenic mice demonstrated that the tumor belonged to pancreatic cystic neoplasm.
CONCLUSION: SV40Tag transgenic mouse model can be successfully established. The transgenic mice develop a pancreatic tumor, which can be used for investigation of the molecular mechanism of tumorigenesis in vivo.
- Citation: Sun Q, Feng J, Wei XL, Zhang R, Dong SZ, Shen Q, Dong J, Li HD, Hu YH. Generation and characterization of a transgenic mouse model for pancreatic cancer. World J Gastroenterol 2006; 12(17): 2785-2788
- URL: https://www.wjgnet.com/1007-9327/full/v12/i17/2785.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i17.2785
Pancreatic cancer is one of the most common malignant diseases threatening human life. The survival rate of pancreatic cancer patients is among the lowest in all kinds of cancer patients. The 1- 3- and 5-year survival rates have been reported to be 16%, 5% and 4%, respectively[1]. Early diagnosis and effective therapeutic intervention are essential to improve the situation. Animal models of pancreatic cancer should provide important tool for the identification of biomarkers for early detection and molecular mechanism studies of the pathological procession of pancreatic cancer.
Simian virus 40 T antigen (SV40Tag) is a virus oncogene encoded by simian virus 40 (SV40). SV40Tag protein is a multifunctional regulatory protein that binds to and inactivates tumor suppressor genes, including P53 and Rb. It has been shown that interaction between SV40Tag and tumor suppressor proteins stimulates DNA duplication, thus facilitating cell proliferation and inducing cell transformation[2,3]. A number of tumor transgenic animal models have been established, including pancreatic cancer[2,3], bone sarcoma[4], urinary bladder carcinoma[5] and liver cancer[6]. In this report, we described the generation and characterization of a SV40Tag transgenic mouse model for pancreatic cancer. The animal model may be useful for molecular mechanism studies on this disease.
FVB and CD-1 mice were provided by Center of Comparative Medicine of Yangzhou University. Mice were maintained in standard laboratory conditions in a 12h light/12h dark cycle.
A 2482-bp SV40Tag DNA fragment was generated by PCR using COS-1 genomic DNA as template. The PCR product containing the entire SV40Tag coding region and a 346 bp intron with two Xho I sites at both ends was purified, digested with Xho I and then cloned into pBC-1 vector to generate the transgenic construct pBC-SV40Tag.
An 18.2 kb pBC-SV40Tag fragment was purified from agarose gel (Qiagen, CA, USA) and adjusted to a final concentration of 2 μg/mL in PBS. FVB female mice were hormonally super-ovulated and mated with FVB male mice. The fertilized eggs were collected from the oviduct 24 h later. Transgene pBC-SV40Tag in PBS was microinjected into the pronuclei of fertilized eggs. Finally, the injected fertilized eggs were transplanted into the oviduct of pseudo-pregnant CD-1 mice.
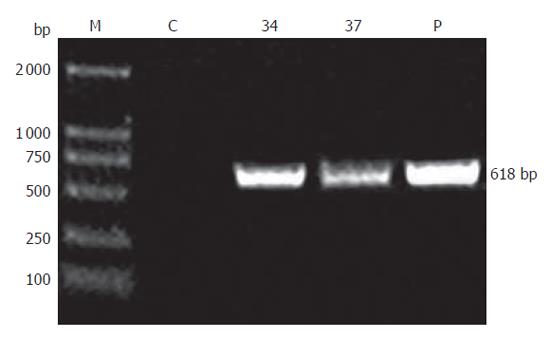
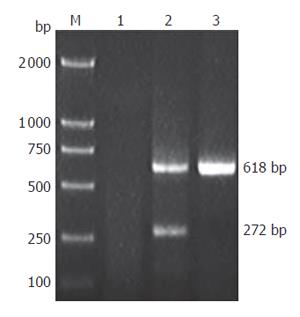
Founder (G0) mice were identified by PCR and Southern blotting analysis. Genomic DNA was isolated from the tails of transgenic mice using a standard method. For PCR, a pair of primers spanning SV40Tag introns was designed (forward: 5’-ACTTTGGAGGCTTCTGGGAT-3’ and reverse: 5’-GGTGTAAATAGCAAAGCAAGCA-3’), which would produce a 618 bp fragment for genomic DNA template and a 272 bp fragment for cDNA template. PCR using genomic DNA as template was performed under the following condition: 35 cycles at 94 °C for 1 min, at 60 °C 45 s, at 72 °C for 50 s. PCR products were detected by electrophoresis on 1.0 % agarose gels. After PCR screening, transgenic mice were further confirmed by Southern blot analysis. Genomic DNA was digested overnight with Nde I and subjected to electrophoresis on a 0.8 % agarose gels. DNA was transferred onto nylon membrane (Millipore Co., Ltd). The 1 kb fragment of plasmid pBC-SV40Tag digested with Nde I was used as a probe. Southern blot analysis was performed according to ECLTM direct nucleic acid labeling and detection system (Amersham).
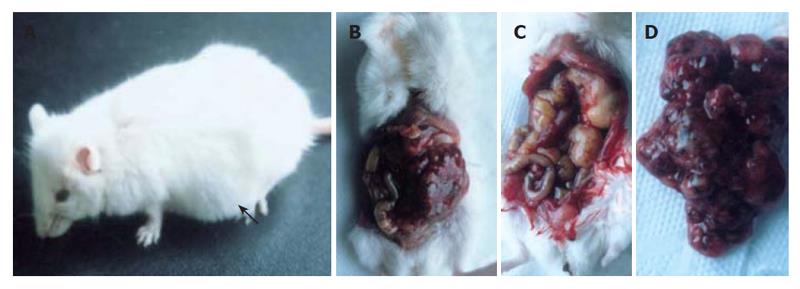
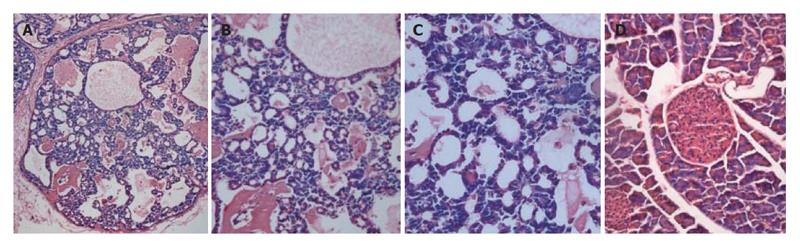
Transgenic mice were examined every day. Mice with bulgy belly and showing listlessness were anatomized and photographed. Various organs and tumors were dissected from the transgenic mice and fixed in 10 % formalin. Sections were obtained from paraffin-embedded tissue samples, stained with hematoxylin-eosin, and examined under a microscope and photographed.
Total RNA was isolated from tumor and other tissues with TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. First strand cDNA was synthesized using reverse transcriptase (Promega). RT-PCR was performed using the SV40Tag primers described above. PCR was performed for 35 cycles at 94 °C for 1 min, at 60 °C for 45 s, and at 72 °C for 50 s. The PCR products were analyzed by electrophoresis on 1.0 % agarose gels.
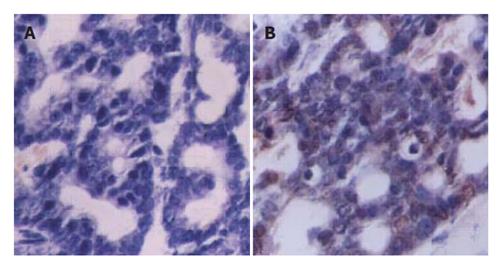
Tissues expressing SV40Tag were collected and fixed in 10 % formalin. Paraffin-embedded tissue samples were cut into 5μm-thick sections. For immunohistochemistry, tissues were de-waxed and treated with newly diluted 3 % H2O2 for 15 min to destroy internal peroxidases. Tissues were incubated with normal goat serum at room temperature for 20 min, then incubated with polyclonal antibody of SV40Tag (Santa cruz biotechnology, sc-20800, 1∶400) at 37 °C for 120 min, and finally with goat anti-rabbit secondary antibodies at 37 °C for 30 min. The samples were stained with DAB using SABC kit (Boster). The nuclei were counterstained with hematoxylin. Slices were observed under microscope and photographed (Olympus).
pBC-SV40Tag was generated and confirmed by restriction enzyme map and DNA sequence analysis. A total of 39 mice were analyzed for production of transgenic mice. Two mice, one male and one female, were identified as transgenic mice by PCR (Figure 1). The serial numbers of the positive mice were line 34 (female) and line 37 (male). These transgenic mice were confirmed by Southern blotting analysis using Nde I digested SV40Tag DNA fragment as a probe (Figure 2). The line 34 developed a tumor at early stage (about 4 wk). Line 37 produced F1 off-springs by mating with normal FVB female mice.
Line 37 mice and positive off-springs developed tumor. At age of 10 wk, the mice showed a palpable abdominal mass and became gradually depressed and moribund (Figure 3A). After the skin of abdomen was dissected, tumor tissue in the abdominal cavity was observed across peritoneum. In addition, a multilocular cystic tumor was identified in the abdominal cavity (Figures 3B and 3C). The diameter of the cysts ranged from 2 mm to 2 cm. The cystic cavities were filled with wine fluid (Figure 3D). Additionally, the spleen and kidney became swollen, and the kidney showed obvious pathological changes. No other obvious pathological changes were observed in other organs (data not shown). The cystic spaces were lined by multi-layer epithelium cells (Figures 4A-4C).
To examine whether the transgene was expressed in multiple tissues and tumor of transgenic mice, we performed RT-PCR for SV40Tag. A 272 bp PCR product was generated from tumor cDNA (Figure 5) by using SV40Tag specific primers, but not in other tissues. Furthermore, immunohistochemical analysis detected SV40Tag protein expression in tumor tissues of transgenic mice (Figure 6).
The early region of simian virus 40 (SV40) encodes a tumor antigen (Tag), a multifunctional regulatory protein responsible for the alterations of cellular processes is required for viral infection[7]. SV40 Tag forms complexes with and inactivates the key cell cycle regulatory proteins, such as p53 and retinoblastoma (Rb) tumor suppressor gene product[7-10]. Loss of these functional tumor suppresso gene products is often associated with human cancer. SV40 Tag expression under the control of tissue specific promoters has been used to generate a variety of transgenic models for human cancers[11-14]. We have used breast-specific β-casein promoter for SV40 Tag transgenic models. Since the β-casein promoter can specifically drive the expression of SV40 Tag in the breast, we expected to generate a breast cancer transgenic mice. Surprisingly, the transgenic mice failed to produce breast cancer but developed pancreatic neoplastic lesions. One possible explanation is that the transgene has been integrated into a particular location of the mouse genome and leads to the expression of SV40 Tag in the pancreas. Another possibility is that the regulated element of the expression vector has been activated in the pancreas. In addition, the transgenic female mice developed a pancreatic tumor and died before lactation. Thus we have no way to know whether SV40 Tag is expressed in the galactophore of the transgenic mice.
Cystic pancreatic neoplasms comprise a heterogeneous group of pathologic entities. Although the incidence rate and lethality of the cystic pancreatic neoplasm are not as high as that of the pancreatic adenocarcinoma, some of the cystic pancreatic neoplasms can develop into metastatic pancreatic adenocarcinoma. The most common cystic pancreatic neoplasms are mucinous cystic neoplasm and serous cystadenoma. These two entities account for more than 75% of the cystic pancreatic neoplasms. Other less common cystic pancreatic neoplasms include papillary cystic tumor (also known as solid and papillary tumor), cystic neuroendocrine tumor, acinar cystadenocarcinoma, cystic teratoma, lymphangioma, hemangioma, and paraganglioma[15]. Due to the low incidence and recent nosographic arrangement, the natural evolution and the clinical development of these neoplasms are not well known. It is, therefore, difficult to identify all types of these cystic lesions. Pancreatic cancers are rarely observed spontaneously or induced by carcinogen administration in the laboratory mice. However, genetic engineering has been shown to effectively generate mice with germline oncogenic lesions similar to human pancreatic adenocarcinomas[16]. We have generated a transgenic animal model with classic features of human cystic pancreatic cancer. This model provides a tractable genetic system to dissect the complexities of evolving cancers in a physiological context. We believe that the mouse model is helpful for the identification of early disease markers and effective therapeutic targets for the disease.
S- Editor Wang J L- Editor Wang XL E- Editor Liu WF
| 1. | Faivre J, Forman D, Esteve J, Obradovic M, Sant M. Survival of patients with primary liver cancer, pancreatic cancer and biliary tract cancer in Europe. Eur J Cancer. 1998;34:2184-2190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Levine DS, Sanchez CA, Rabinovitch PS, Reid BJ. Formation of the tetraploid intermediate is associated with the development of cells with more than four centrioles in the elastase-simian virus 40 tumor antigen transgenic mouse model of pancreatic cancer. Proc Natl Acad Sci USA. 1991;88:6427-6431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 96] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Powers AC, Efrat S, Mojsov S, Spector D, Habener JF, Hanahan D. Proglucagon processing similar to normal islets in pancreatic alpha-like cell line derived from transgenic mouse tumor. Diabetes. 1990;39:406-414. [PubMed] [DOI] [Full Text] |
| 4. | Marton I, Johnson SE, Damjanov I, Currier KS, Sundberg JP, Knowles BB. Expression and immune recognition of SV40 Tag in transgenic mice that develop metastatic osteosarcomas. Transgenic Res. 2000;9:115-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Grippo PJ, Sandgren EP. Highly invasive transitional cell carcinoma of the bladder in a simian virus 40 T-antigen transgenic mouse model. Am J Pathol. 2000;157:805-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Paul D, Hohne M, Pinkert C, Piasecki A, Ummelmann E, Brinster RL. Immortalized differentiated hepatocyte lines derived from transgenic mice harboring SV40 T-antigen genes. Exp Cell Res. 1988;175:354-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 65] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Dobbelstein M, Arthur AK, Dehde S, van Zee K, Dickmanns A, Fanning E. Intracistronic complementation reveals a new function of SV40 T antigen that co-operates with Rb and p53 binding to stimulate DNA synthesis in quiescent cells. Oncogene. 1992;7:837-847. [PubMed] |
| 8. | DeCaprio JA, Ludlow JW, Figge J, Shew JY, Huang CM, Lee WH, Marsilio E, Paucha E, Livingston DM. SV40 large tumor antigen forms a specific complex with the product of the retinoblastoma susceptibility gene. Cell. 1988;54:275-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1039] [Cited by in RCA: 1090] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 9. | Wilkie TM, Schmidt RA, Baetscher M, Messing A. Smooth muscle and bone neoplasms in transgenic mice expressing SV40 T antigen. Oncogene. 1994;9:2889-2895. [PubMed] |
| 10. | Conzen SD, Cole CN. The three transforming regions of SV40 T antigen are required for immortalization of primary mouse embryo fibroblasts. Oncogene. 1995;11:2295-2302. [PubMed] |
| 11. | Faas SJ, Pan S, Pinkert CA, Brinster RL, Knowles BB. Simian virus 40 (SV40)-transgenic mice that develop tumors are specifically tolerant to SV40 T antigen. J Exp Med. 1987;165:417-427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Murphy D, Bishop A, Rindi G, Murphy MN, Stamp GW, Hanson J, Polak JM, Hogan B. Mice transgenic for a vasopressin-SV40 hybrid oncogene develop tumors of the endocrine pancreas and the anterior pituitary. A possible model for human multiple endocrine neoplasia type 1. Am J Pathol. 1987;129:552-566. [PubMed] |
| 13. | Patrick TA, Kranz DM, van Dyke TA, Roy EJ. Folate receptors as potential therapeutic targets in choroid plexus tumors of SV40 transgenic mice. J Neurooncol. 1997;32:111-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Calvo A, Yokoyama Y, Smith LE, Ali I, Shih SC, Feldman AL, Libutti SK, Sundaram R, Green JE. Inhibition of the mammary carcinoma angiogenic switch in C3(1)/SV40 transgenic mice by a mutated form of human endostatin. Int J Cancer. 2002;101:224-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Rattner DW, Fernandez-del Castillo C, Warshaw AL. Cystic pancreatic neoplasms. Ann Oncol. 1999;10 Suppl 4:104-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Aguirre AJ, Bardeesy N, Sinha M, Lopez L, Tuveson DA, Horner J, Redston MS, DePinho RA. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev. 2003;17:3112-3126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 772] [Cited by in RCA: 819] [Article Influence: 37.2] [Reference Citation Analysis (0)] |