Published online May 7, 2006. doi: 10.3748/wjg.v12.i17.2756
Revised: October 20, 2006
Accepted: November 10, 2005
Published online: May 7, 2006
AIM: To compare ecabet sodium and cimetidine in relieving symptoms of functional dyspepsia.
METHODS: We performed a multi-center, prospective, randomized, double-blinded controlled trial to compare the clinical efficacy of ecabet sodium and cimetidine in patients with functional dyspepsia. Two-hundred and seventy-two patients with dyspeptic symptoms fulfilling the Rome-II criteria were enrolled from 7 centers. In the study group (115 patients), 1.5 g ecabet sodium was given twice a day. In the control group (121 patients), 400 mg cimetidine was given twice a day. Symptoms and parameters of quality of life were analyzed at baseline, 3, 14, and 28 d after initiating the treatment.
RESULTS: Two-hundred and thirty-six patients completed the clinical trial. After 4 wk of treatment, the rates of improvement in patients with dyspeptic symptoms were not different between two groups (77.4% in the ecabet group and 79.3% in the cimetidine group, respectively, P > 0.05). Likewise, the rates of symptomatic improvement were not different at 3 d and 14 d. The parameters of quality of life did not change significantly during the study period in both groups. There was no clinically significant adverse event in both groups.
CONCLUSION: In patients with functional dyspepsia, ecabet sodium has similar clinical efficacy with cimetidine.
- Citation: Lee JH, Kim JJ, Hahm KB, Lee DH, Kim N, Kim SK, Park JJ, Choi SR, Lee JH, Lee ST, Lee EH, Rhee JC. Efficacy and safety of ecabet sodium on functional dyspepsia: A prospective, double-blinded, randomized, multi-center controlled trial. World J Gastroenterol 2006; 12(17): 2756-2761
- URL: https://www.wjgnet.com/1007-9327/full/v12/i17/2756.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i17.2756
Functional dyspepsia is defined as persistent or recurrent abdominal pain or abdominal discomfort centered in the upper abdomen which cannot be explained by structural or biological abnormalities[1,2]. Discomfort refers to unpleasant sensations that the subject does not interpret as pain and may be characterized by upper abdominal fullness, early satiety, bloating, belching, or nausea. For the diagnosis of functional dyspepsia, there should be no relationship between dyspeptic symptoms and bowel movements (i.e., irritable bowel syndrome does not explain the symptoms), and patients with predominant heartburn should be excluded.
At present, the pathophysiology of functional dyspepsia is not fully elucidated. Several mechanisms have been suggested to explain the development of dyspeptic symptoms, including delayed gastric emptying[3], impaired gastric accommodation to a meal[4], hypersensitivity to gastric distention[5,6], Helicobacter pylori (H pylori) infection[7], and central or peripheral nervous system dysfunction[8]. However, there is growing evidence that functional dyspepsia is in fact a very heterogeneous disorder and different subgroups can be identified based on different demographic, clinical, and pathophysiologic features[2].
Pharmacological treatment for patients with functional dyspepsia remains unsatisfactory. The results of studies with H2-receptor antagonists have been conflicting[9,10]. Proton pump inhibitors appear to be superior to placebo, but not in dysmotility-like dyspepsia[11]. Cisapride has also been demonstrated to be effective[12], but it has been reported that cisapride is not superior to placebo[13]. Therefore, it seems likely that acid-suppressing agents and prokinetic agents are, at best, only effective in some subgroups of dyspeptic patients.
Ecabet sodium (ES), a 12-sulfodehydroabietic acid monosodium salt, is a novel non-systemic anti-ulcer agent[14-16] and belongs to the category of gastroprotective agents[17]. The utility of ES in clinical setting has been demonstrated in various clinical trials for patients with peptic ulcer, ulcerative proctosigmoiditis, and H pylori infection[18-21]. In this prospective randomized controlled study, we attempted to assess the efficacy and safety of ES in patients with functional dyspepsia.
From October 2003 to September 2004, this trial was performed at 7 centers in Korea. A total of 272 patients with functional dyspepsia according to Rome-II criteria were enrolled. Upper gastrointestinal endoscopy was performed to exclude a structural cause of the symptoms. All patients understood the purpose and method of this study and agreed to this clinical trial with written informed consent.
Exclusion criteria included age under 18 years or over 75 years, pregnancy or lactation, regular use of steroids or NSAIDs, an experience in administration of any drug for treating the subject disease, including gastric acid secretion inhibitors (proton pump inhibitor, H2-blocker, etc.) within 4 wk of enrollment. Patients demonstrating gastric ulcer or duodenal ulcer on upper gastrointestinal endoscopy, were excluded from this study. Other exclusion criteria included gastroesophageal reflux disease with typical symptoms like heartburn or acid regurgitation, malignant diseases, and serious hepatic, cardiovascular, renal, or hematological diseases. Patients who were unable to answer the visual analogue scale question due to poor intellectual power were also excluded.
A prospective, double-blinded, randomized, comparative trial was performed in 7 centers. Patients were randomized to receive either ecabet sodium (1.5 g p.o, bid) or cimetidine (400 mg p.o, bid) for 4 wk. All medications were given within 30 minutes after breakfast and just before going to bed.
At screening, upper gastrointestinal endoscopy, complete blood cell counts, blood chemistries and serology for H pylori infection were performed. If the result of upper gastrointestional endoscopy within 3 mo of the enrollment was not significant, repeat endoscopic examination was not performed. At the beginning of the study, patients were required to give written informed consent and to answer the questionnaire for the evaluation of the quality of life and for individual dyspeptic symptoms. Assessment of the quality of life using the same questionnaire was repeated at the end of the treatment.
The symptoms were evaluated 4 times (baseline, 3, 14, and 28 d) using index of dyspepsia symptoms-Koreans (IDS-K) questionnaire validated in Korea[22]. This questionnaire format was designed to evaluate 12 typical upper gastrointestinal symptoms using 10-cm visual analogue scale. In addition, patients were asked to judge the global improvement of symptoms as significant improvement, somewhat improvement, no change, somewhat worsen, and severely worsen. In order to measure the overall efficacy of the medication, treatment effect was categorized into 2 groups. Significant improvement and somewhat improvement were judged as improved, while no change, somewhat worsen and severely worsen were judged as not improved.
For the evaluation of the quality of life, FD-QoL questionnaire was completed in coordinator’s interview before and at the end of the treatment. The questionnaire was consisted of 21 items of 4 dimensions (5 questions for the eating status, 4 questions for the liveliness status, 6 questions for the psychologic status, and 6 questions for the role-functioning status). Higher scores indicated better quality of life. The sum of scores was calculated and compared to assess the quality of life before and after the treatment. Safety of the medication was evaluated by the assessment of significant adverse events. At the end of the treatment, complete blood cell counts and blood chemistries were repeated.
Efficacy data analyses were performed based on per-protocol analysis set. Clinical characteristics of target patients were summarized with technical statistics. Consecutive variables were described with mean ± SD and the categorical variables with proportion. With respect to the global improvement of symptoms, effective group and non-effective group were classified and compared using χ2-test. With respect to the quality of life, a paired t-test was used to compare the difference of the sum of scores by each dimension between before-treatment and end-of-treatment. Additionally, subgroup analyses of the symptom score and the score of quality of life were conducted according to age, infection of H pylori and sex.
A total number of 272 patients (133 patients in ecabet group, 139 patients in cimetidine group) with functional dyspepsia were enrolled. In both groups, there was no significant difference in demographic characteristics including age, sex, weight, height, rate of smokers, and rate of alcohol intake (Table 1). Thirty-six patients (18 patients in ecabet group, 18 patients in cimetidine group) of them were dropped out. A total of 236 patients (115 patients in ecabet group, 121 patients in cimetidine group) were included in the per-protocol analysis. There was no significant difference between two groups in medication compliance 2 and 4 wk after administration. There was no significant difference between treatment groups with the respect to the proportion of patients with H pylori infection (Table 2).
| Group | Ecabet group | Cimetidine group | P |
| (n = 133) | (n = 139) | ||
| Gender (male:female) | 47:86 | 47:92 | NS |
| Age (yr) | 45.0 ± 12.9 | 47.5 ± 12.5 | NS |
| Weight (kg) | 59.6 ± 10.0 | 58.5 ± 9.0 | NS |
| Height (cm) | 162.7 ± 8.0 | 161.8 ± 7.7 | NS |
| Smoking (%) | 23 (17.3) | 16 (11.5) | NS |
| Alcohol intake (%) | 47 (35.3) | 38 (27.3) | NS |
| Group | Positive (%) | Negative (%) | Undetermined (%) |
| Ecabet group | 44 | 45 | 31 |
| (n = 120) | (36.7) | (37.5) | (25.9) |
| Cimetidine group | 40 | 60 | 28 |
| (n = 128) | (31.3) | (46.9) | (21.9) |
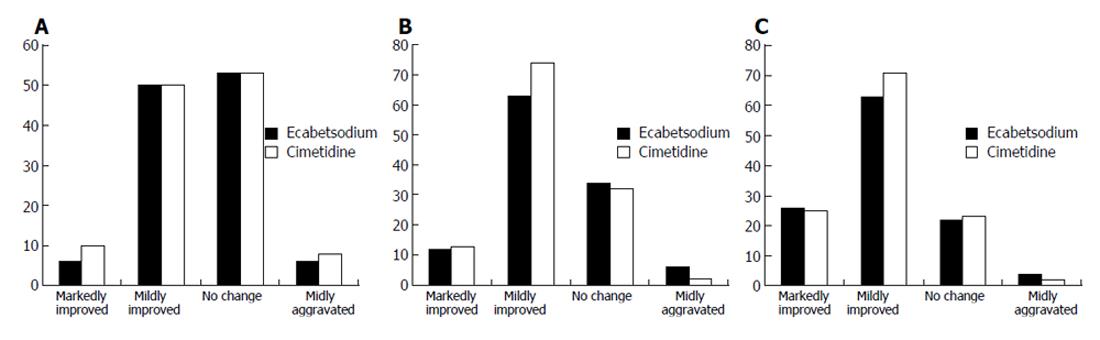
For the measurement of the overall rate of improvement, a question for global symptomatic assessment was used. The result of evaluation of symptoms at baseline, 3 d, 14 d and 28 d after treatment demonstrated no significant difference between the groups (Figures 1A-C). However, both groups showed significant improvement of symptoms at 2 and 4 wk compared to the baseline (Figures 1B, IC).
When significant improvement and somewhat improvement were considered as improved, and no change, somewhat worsen, and severely worsen as not improved, the rate of improvement at 4 wk was not different between ecabet group and cimetidine group (Table 3) (P = 0.53). The sum of 12 symptom scores of both groups was not different at 3 d, 2 wk and 4 wk (P = 0.84).
| Group | Ecabet group | Cimetidine group |
| (n = 115) (%) | (n = 121) (%) | |
| Improved | 89 (77.4) | 96 (79.3) |
| Not improved | 26 (22.6) | 25 (20.7) |
The parameters of quality of life were categorized into eating status, liveliness status, psychological status, and role-functioning status. In both groups, the sum of quality of life scores in each category did not change significantly after treatment (Table 4).
| Status | Before treatment | After treatment | ||||
| Ecabet group(n = 115) | Cimetidine group(n = 121) | P | Ecabet group(n = 115) | Cimetidine group(n = 121) | P | |
| Eating status | 15.0 ± 4.6 | 14.8 ± 4.1 | NS | 15.6 ± 4.0 | 15.9 ± 3.5 | NS |
| Liveliness status | 10.4 ± 4.1 | 10.0 ± 4.0 | NS | 11.6 ± 3.6 | 11.3 ± 3.5 | NS |
| Psychological status | 18.5 ± 5.0 | 18.6 ± 4.5 | NS | 20.3 ± 4.4 | 20.4 ± 4.1 | NS |
| Role-functioning status | 20.5 ± 4.2 | 20.6 ± 4.2 | NS | 21.4 ± 3.9 | 21.0 ± 4.3 | NS |
After treatment, there was no significant change in laboratory parameters such as WBC count per mm3, ESR, and CRP in both groups.
The patients were grouped by serologic result with H pylori-positive group and -negative group. With the respect to the global improvement of symptoms, there was no significant difference between two groups regardless of H pylori infection (Table 5). In the subgroups by the age, categorized with less than 50 years old and equal/over 50 years old groups, there was no difference in the global symptom improvement in both groups (Table 6). In the subgroups by the gender, there was also no difference in the global symptom improvement in both groups (Table 7).
| H pylori infection | Gastrex group | Cimetidine group | ||
| Improved (%) | Not improved(%) | Improved (%) | Not improved (%) | |
| Negative | 28 (84.85) | 5 (15.15) | 27 (84.38) | 5 (15.63) |
| Positive | 28 (75.68) | 9 (24.32) | 24 (72.73) | 9 (27.27) |
| Age | Gastrex group | Cimetidine group | ||
| Improveed (%) | Not improved (%) | Improved (%) | Not improved (%) | |
| Less than fifty | 55 (61.8) | 14 (53.85) | 61 (63.54) | 12 (48.0) |
| fifty and over | 34 (38.2) | 12 (46.15) | 35 (36.46) | 13 (52.0) |
| Sex | Gastrex group | Cimetidine group | ||
| Improved (%) | Not improved (%) | Improved (%) | Not improved (%) | |
| Men | 27 (71.05) | 11 (28.94) | 34 (79.07) | 9 (20.93) |
| Women | 63 (80.76) | 15 (19.23) | 62 (79.48) | 16 (20.51) |
There was no severe adverse event during the study period. Three patients among 133 patients in the ecabet group reported mild adverse events, such as diarrhea, rash and abdominal pain. However, the events were treated with simple symptomatic treatments. Two patients among 139 patients in the cimetidine group complained of mild diarrhea. In the chemistry at 4 wk, mild transient elevation of transaminases occurred in 1 case in the ecabet group and 1 case in the cimetidine group, respectively.
Functional dyspepsia is a symptom complex characterized by postprandial upper abdominal discomfort or pain, early satiety, nausea, vomiting, abdominal distention, bloating, and anorexia in the absence of organic disease. In this study, we used the Rome-II criteria for the exact identification of patients with functional dyspepsia[1].
The major pathophysiological mechanisms responsible for functional dyspepsia include psychosocial factors and alterations in motility and visceral perception. Approximately 50% of patients with functional dyspepsia have motor disorders, such as impaired fundic relaxation, antral dilatation and/or hypomotility, gastroparesis. It is also observed that symptoms of functional dyspepsia are related to or worsen by several psychological factors, such as negative feelings as anxiety and/or depression, abnormal stress response, dependence personality, change of coping strategy, etc[2-4]. The important mental element related with patients with functional dyspepsia is stress of daily life. At present, administration of antidepressants has been found to be useful for only a few patients, control of psycho-sociable element is not assessed yet[2].
Ecabet sodium (ES) is a newly developed anti-inflammatory/anti-ulcer agent and belongs to the category of gastroprotective agents[14-16]. Proposed mechanisms of action include improved blood flow in the gastric mucosa, increased gastric mucin, increased gastric mucosal PG E2, and decreased activity of pepsin[15,19]. It was reported that ES increases the eradication rate of Helicobacter pylori in dual therapy with lansoprazole and amoxicillin[18]. In patients with gastric ulcer treated with cimetidine, additional use of ES significantly accelerates rates of ulcer healing and symptomatic relief[19]. Maintenance therapy with a combination of ranitidine and ES prevents ulcer relapse in Helicobacter pylori-positive patients[20]. Interestingly, ES enemas have been proved to be a safe and potentially useful adjuvant therapy in patients with mildly to moderately active ulcerative proctosigmoiditis[21].
There are only a few clinical studies examining the effect of mucosal protective agents in patients with functional dyspepsia. A double-blind placebo-controlled multicenter study examining the effect of rabamipide in patients with functional dyspepsia reported that there is no significant improvement of individual symptom scores in rebamipide group compared to placebo group[23]. However, the efficacy of ES for patients with functional dyspepsis has never been reported in the literature. In this study examining the efficacy of ES in patients with functional dyspepsia, ES showed equivalent outcomes in most clinical indexes compared to a representative H2 receptor antagonist (cimetidine). Rates of improvement of global symptoms were not different at 3 d, 14 d, and 28 d after starting treatment (Table 3). At the end of the 4-wk treatment, 77.4% of patients in ecabet group and 79.3% of patients in the cimetidine group reported improvement of global symptoms (P > 0.05). In the analysis of 12 individual symptoms, there was no significant difference in both groups. In the subgroup analysis by age, gender and H pylori infection status, we could not find any factors affecting the rate of improvement.
Health-related quality of life is becoming increasingly recognized as an important outcome for patients with chronic diseases[24]. Patients with functional dyspepsia can experience significant levels of abdominal pain that interrupt daily activities, so quality of life is impaired[25]. In patients with functional dyspepsia, therefore, the impact of treatment on the quality of life can be a good measure of treatment outcome. For the evaluation of the quality of life, we used the IDS-K questionnaire, which was recently developed and validated in Korea[22]. Despite improvement of dyspeptic symptoms during the treatment period, we could not find any significant change in any dimension of our questionnaire before and after the 4-wk treatment (Table 4). One possible reason for this discrepancy may be that the duration of 4-wk treatment may be too short to document a significant change in quality of life. Longer-term treatment may be necessary for the exact evaluation of the impact of treatment on the quality of life in dyspeptic patients.
The major limitation of the present study is the lack of treatment arm using placebo. In this study, we could see a significant improvement of symptoms in both ecabet group and cimetidine group. The reported response rates of cimetidine for patients with non-ulcer dyspepsia are quite variable. Nyren et al[26] reported that the mean reduction in pain intensity after three weeks is less than 30% in both placebo and cimetidine group. In a meta-analysis of placebo-controlled studies for non-ulcer dyspepsia, the mixed response rate for cimetidine treatment is 67.3% (272/404)[27]. In the present study, the response rate of ecabet group was 77.4%, and that of cimetidine group was 79.3% (Table 3). The rate of improvement in this study seemed to be a little bit higher than that in the previous studies. In our opinion, differences in the patient population and the definition of improvement may be possible explanations. In the present study, there was no arm for the placebo, so we could not exclude the possibility that the outcome of this study might be a placebo effect. In the previously mentioned meta-analysis, however, the mixed response rate for placebo is 49.1% (183/372)[25]. In order to determine the efficacy of ES for functional dyspepsia more exactly, long-term randomized clinical trial with placebo arm is mandatory.
In conclusion, 4-wk treatment with ES demonstrates equivalent symptom improvement compared to a representative H2-receptor antagonist (cimetidine) in patients with functional dyspepsia.
S- Editor Guo SY L- Editor Wang XL E- Editor Liu WF
| 1. | Talley NJ, Stanghellini V, Heading RC, Koch KL, Malagelada JR, Tytgat GN. Functional gastroduodenal disorders. Gut. 1999;45 Suppl 2:II37-II42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 415] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 2. | Tack J, Bisschops R, Sarnelli G. Pathophysiology and treatment of functional dyspepsia. Gastroenterology. 2004;127:1239-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 337] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 3. | Quigley EM. Review article: gastric emptying in functional gastrointestinal disorders. Aliment Pharmacol Ther. 2004;20 Suppl 7:56-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 4. | Caldarella MP, Azpiroz F, Malagelada JR. Antro-fundic dysfunctions in functional dyspepsia. Gastroenterology. 2003;124:1220-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 106] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Fischler B, Tack J, De Gucht V, Shkedy ZI, Persoons P, Broekaert D, Molenberghs G, Janssens J. Heterogeneity of symptom pattern, psychosocial factors, and pathophysiological mechanisms in severe functional dyspepsia. Gastroenterology. 2003;124:903-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 101] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 6. | Perri F, Festa V, Grossi E, Garbagna N, Leandro G, Andriulli A. Dyspepsia and Helicobacter pylori infection: a prospective multicentre observational study. Dig Liver Dis. 2003;35:157-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Rhee PL, Kim YH, Son HJ, Kim JJ, Koh KC, Paik SW, Rhee JC, Choi KW. Evaluation of individual symptoms cannot predict presence of gastric hypersensitivity in functional dyspepsia. Dig Dis Sci. 2000;45:1680-1684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Park DI, Rhee PL, Kim YH, Sung IK, Son HJ, Kim JJ, Paik SW, Rhee JC, Choi KW. Role of autonomic dysfunction in patients with functional dyspepsia. Dig Liver Dis. 2001;33:464-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Farup PG, Wetterhus S, Osnes M, Ulshagen K. Ranitidine effectively relieves symptoms in a subset of patients with functional dyspepsia. Scand J Gastroenterol. 1997;32:755-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Redstone HA, Barrowman N, Veldhuyzen Van Zanten SJ. H2-receptor antagonists in the treatment of functional (nonulcer) dyspepsia: a meta-analysis of randomized controlled clinical trials. Aliment Pharmacol Ther. 2001;15:1291-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Talley NJ, Meineche-Schmidt V, Pare P, Duckworth M, Raisanen P, Pap A, Kordecki H, Schmid V. Efficacy of omeprazole in functional dyspepsia: double-blind, randomized, placebo-controlled trials (the Bond and Opera studies). Aliment Pharmacol Ther. 1998;12:1055-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 261] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 12. | Holtmann G, Gschossmann J, Mayr P, Talley NJ. A randomized placebo-controlled trial of simethicone and cisapride for the treatment of patients with functional dyspepsia. Aliment Pharmacol Ther. 2002;16:1641-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Champion MC, MacCannell KL, Thomson AB, Tanton R, Eberhard S, Sullivan SN, Archambault A. A double-blind randomized study of cisapride in the treatment of nonulcer dyspepsia. The Canadian Cisapride Nud Study Group. Can J Gastroenterol. 1997;11:127-134. [PubMed] |
| 14. | Kinoshita M, Yamasaki K, Kokusenya Y, Tamaki H. Relationship between gastroprotective effect of locally acting antiulcer agent ecabet sodium and its binding to gastric mucosa in rats. Comparison with sucralfate. Dig Dis Sci. 1995;40:661-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Ichikawa T, Ishihara K, Hayashida H, Hiruma H, Saigenji K, Hotta K. Effects of ecabet sodium, a novel gastroprotective agent, on mucin metabolism in rat gastric mucosa. Dig Dis Sci. 2000;45:606-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Kinoshita M, Tamaki H. Possible mechanism of increase in gastric mucosal PGE2 and PGI2 generation induced by ecabet sodium, a novel gastroprotective agent. Dig Dis Sci. 1997;42:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Slomiany BL, Murty VL, Piotrowski J, Slomiany A. Gastroprotective agents in mucosal defense against Helicobacter pylori. Gen Pharmacol. 1994;25:833-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Kagaya H, Kato M, Komatsu Y, Mizushima T, Sukegawa M, Nishikawa K, Hokari K, Takeda H, Sugiyama T, Asaka M. High-dose ecabet sodium improves the eradication rate of helicobacter pylori in dual therapy with lansoprazole and amoxicillin. Aliment Pharmacol Ther. 2000;14:1523-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Murata H, Kawano S, Tsuji S, Kamada T, Matsuzawa Y, Katsu K, Inoue K, Kobayashi K, Mitsufuji S, Bamba T. Combination therapy of ecabet sodium and cimetidine compared with cimetidine alone for gastric ulcer: prospective randomized multicenter study. J Gastroenterol Hepatol. 2003;18:1029-1033. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Koizumi W, Tanabe S, Imaizumi H, Kida M, Ohida M, Koshida Y, Mitomi H, Hosaka Y, Nagaba S, Sasaki T. Inhibition of peptic ulcer relapse by ranitidine and ecabet independently of eradication of Helicobacter pylori: a prospective, controlled study versus ranitidine. Hepatogastroenterology. 2003;50:577-581. [PubMed] |
| 21. | Kono T, Nomura M, Kasai S, Kohgo Y. Effect of ecabet sodium enema on mildly to moderately active ulcerative proctosigmoiditis: an open-label study. Am J Gastroenterol. 2001;96:793-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Lee EH, Hahm KB, Lee JH, Park JJ, Lee DH, Kim SK, Choi SR, Lee ST. Development and validation of a functional dyspepsia-related quality of life (FD-QOL) scale in South Korea. J Gastroenterol Hepatol. 2006;21:268-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Talley NJ, Riff DS, Schwartz H, Marcuard SP. Double-blind placebo-controlled multicentre studies of rebamipide, a gastroprotective drug, in the treatment of functional dyspepsia with or without Helicobacter pylori infection. Aliment Pharmacol Ther. 2001;15:1603-1611. [RCA] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | El-Serag HB, Talley NJ. Health-related quality of life in functional dyspepsia. Aliment Pharmacol Ther. 2003;18:387-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 117] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 25. | Talley NJ, Verlinden M, Jones M. Quality of life in functional dyspepsia: responsiveness of the Nepean Dyspepsia Index and development of a new 10-item short form. Aliment Pharmacol Ther. 2001;15:207-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 98] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 26. | Nyrén O, Adami HO, Bates S, Bergström R, Gustavsson S, Lööf L, Nyberg A. Absence of therapeutic benefit from antacids or cimetidine in non-ulcer dyspepsia. N Engl J Med. 1986;314:339-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 120] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Allescher HD, Bockenhoff A, Knapp G, Wienbeck M, Hartung J. Treatment of non-ulcer dyspepsia: a meta-analysis of placebo-controlled prospective studies. Scand J Gastroenterol. 2001;36:934-941. [RCA] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |