Published online Apr 28, 2006. doi: 10.3748/wjg.v12.i16.2584
Revised: January 14, 2006
Accepted: January 24, 2006
Published online: April 28, 2006
AIM: To conduct a retrospective study to determine the risk factors for development of metaplastic gastritis in Korean population.
METHODS: The database of 113 449 subjects who underwent a gastroscopy for the purpose of a regular check-up at center for health promotion, Samsung medical center during 5 years was collected and retrospectively analyzed. Among them, 5 847 subjects who had endoscopically diagnosed as a metaplastic gastritis or 10 076 normal as well as answered to questionnaire were included for present study. The subjects were divided into 2 groups; Group I, normal and Group II, metaplastic gastritis. Age, gender, Helicobacter pylori (H pylori) seropositivity, body mass index (BMI), family history of cancer, smoking, alcohol consumption, total daily calories, folate and salt intake and dietary habit (out-eating, overeating, irregular eating) were retrieved from questionnaire or electronic medical record and compared between group I and group II.
RESULTS: The prevalence of group II was 11% (13 578/113 449) increasing its prevalence with age (P = 0.000). But, there was no significant association between 2 groups in BMI, family history of cancer, alcohol consumption, total daily calories, folate and salt intake and dietary habit (out-eating, overeating, irregular eating). Old age (P = 0.000), male gender (P = 0.000), H pylori seropositivity (P = 0.010) and current smoker (P = 0.000) were significantly more common in group II at multiple logistic regression model.
CONCLUSION: Our data suggested that old age, male gender, H pylori seropositivity and smoking were risk factors for metaplastic gastritis, precancerous lesion of gastric cancer.
- Citation: Choi S, Lim YJ, Park SK. Risk factor analysis for metaplastic gastritis in Koreans. World J Gastroenterol 2006; 12(16): 2584-2587
- URL: https://www.wjgnet.com/1007-9327/full/v12/i16/2584.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i16.2584
Gastric cancer was recognized as the first leading cause of cancer death in Korea[1] and attention turned to epidemiologic associations between risk factors and gastric cancer. In the early 1970s, Correa formulated a multi-step model of gastric cancer, which postulated a temporal sequence of pathologic changes that led from chronic gastritis to atrophic gastritis, intestinal metaplasia and dysplasia and the eventual development of gastric cancer[2]. Chronic gastric inflammation seems to be the critical common cause of gastric cancer[3].
The purpose of this paper was to determine the risk factors for development of metaplastic gastritis, precursor of gastric cancer in Korean population
The database of 113 449 subjects who underwent a gastroscopy for the purpose of a regular check-up at center for health promotion, Samsung medical center from January 2001 through June 2004 was collected and retrospectively analyzed. Among them, 13 578 subjects were endoscopically diagnosed as a metaplastic gastritis and 10 521 subjects was endoscopically diagnosed as a normal. But, only 5 847 subjects who had endoscopically diagnosed as a metaplastic gastritis were answered to questionnaire whereas 10 076 subjects who had endoscopically diagnosed as a normal were answered to questionnaire. Both 5 847 metaplastic gastritis and 10 076 normal were included for present study. Subjects with peptic ulcer or erosion of stomach were excluded in this study population. The subjects were divided into 2 groups; Group I, normal and Group II, metaplastic gastritis. Demographic data [age, gender, body mass index (BMI), family history of cancer] and life style data [smoking, alcohol consumption, total daily calorie intake, folate intake, salt intake, dietary habit (out-eating, overeating, irregular eating)] were retrieved from questionnaire or electronic medical records and compared between group I and group II. Those who have family history of any cancer were those who had a reply to yes in the question (“Do you have a blood relation who has a experience of diagnosis as cancer by a doctor”). Data including smoking, alcohol consumption (frequency and amount of alcohol), dietary habit (out-eating, overeating, irregular eating) were collected from self administered questionnaire. Patients were asked whether they were active, past or never smoker. The subjects were divided into two groups (current smoking group and non-smoking group) by current smoking status. Nonsmoking group is composed of past or non-smoking. The subjects were divided into two groups by frequency and amounts of liquors (namely, so-ju) consumption in questionnaire. Over or equal 3-4 frequencies a week and ≥ 80 g once alcohol consumption is defined as the group of heavy alcohol consumption. Below 3-4 frequencies a week or < 80 g once alcohol consumption is classified as the other group. Total amount of calorie, folate intake and fat intake were obtained in diet surveys. Helicobacter pylori (H pylori) seropositivity was retrieved from electronic medical record. H pylori infection was determined by measuring serum H pylori IgG antibodies. Specific anti- H pylori antibodies were measured with an enzyme-linked immunosorbent assay (ELISA) kit using an antigen (RADIM SpA, Pomezia, Italy). The sensitivity and specificity of this assay was reported to be 79% and 83%, respectively[4].
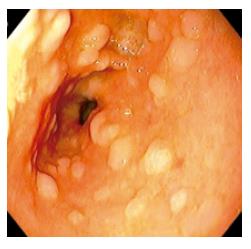
Atrophic mucosa with surface nodularity on the endoscopic finding was diagnosed as metaplastic gastritis (Figure 1). 3 354 individuals who showed endoscopically metaplastic gastritis had also a presence of metaplasia histologically on the updated Sydney system.
Logistic regression analysis was conducted by fixing the each group as a dependent variable and risk factors as independent variables. Continuous variables such as age, BMI, total daily calorie intake, folate intake and salt intake were analyzed by t-test. Gender, smoking, alcohol consumption, family history of cancer, dietary habit (out-eating, overeating, irregular eating) and H pylori seropositivity were analyzed by χ2 test. The relative risk to develop metapalstic gastritis was calculated with odds ratio with 95% confidence interval. Risk factors were examined by multiple logistic regression analysis. Statistical significance was assumed at P < 0.05. Statistical analyses were performed with SAS version 8.1 (SAS Institute Inc, Cary, NC, USA).
| Normal (%) | Metaplastic gastritis (%) | P value | |
| Age | 42.46 ± 10.15 | 53.20 ± 9.00 | 0.000 |
| Sex | 0.000 | ||
| Men | 3804 (36) | 3983 (68) | |
| Women | 6272 (64) | 1864 (32) | |
| BMI1 | 24.52 ± 2.36 | 23.97 ± 2.86 | 0.208 |
| Family history of cancer | 160 (1) | 125 (2) | 0.065 |
| Smoking | 0.000 | ||
| None or past | 2933(84) | 3308 (74) | |
| Current | 550 (16) | 1130 (26) | |
| Alcohol | 0.059 | ||
| Heavy alcoholics2 | 553 (15) | 747 (16) | |
| Out-eating | 2477 (23) | 1535 (26) | 0.110 |
| Over eating | 1743 (16) | 912 (15) | 0.598 |
| Irregular eating | 1824 (17) | 1152 (19) | 0.228 |
| Total calories | 2102.94 ± 470.17 | 2226.46 ± 486.22 | 0.515 |
| Folate | 246.34 ± 91.24 | 289.51 ± 108.55 | 0.211 |
| Salts | 22.48 ± 7.27 | 23.02 ± 4.97 | 0.319 |
| H pylori seropositivity3 | 4165 (39) | 3566 (60) | 0.007 |
The prevalence of group II was 11% (13 578/113 449) increasing its prevalence with age (P = 0.000). But, there was no significant association between 2 groups in BMI, alcohol consumption and family history of cancer. Male gender was a risk factor for metaplastic gastritis and current smokers were more likely to have metaplastic gastritis than none or past smokers. Neither dietary composition (folate, salts, calories) nor dietary habits (out-eating, overeating, irregular eating) was associated with metaplastic gastritis. H pylori seropositivity was more common in the group II.
Finally, we conducted stepwise multiple logistic regression analysis in which above significant variables were used as independent variables. Number of subjects entered into the stepwise multiple logistic regression model were 4 438 in group II and 3483 in group I. Entered variables were age, gender, BMI, family history of cancer, smoking, alcohol consumption, total daily calorie intake, folate intake, salt intake, dietary habit (out-eating, overeating, irregular eating), and H pylori seropositivity.
Old age (P = 0.000), male gender (P = 0.000), H pylori seropositivity (P = 0.010) and current smoker (P = 0.000) were significantly more common in the group II at multiple logistic regression model.
Gastric cancer was recognized as the first leading cause of cancer death in Korea[1] and many epidemiologic studies about risk factors for gastric cancer were reported[5,6]. So far, we had no a large-scale epidemiologic studies about metaplastic gastritis, precursors of gastric cancer in the Korean population.
In the early 1970s, Correa formulated a multi-step model of gastric cancer, which postulated a sequence from chronic atrophic gastritis, intestinal metaplasia, dysplasia and gastric cancer[2]. Chronic gastric inflammation leads to repetitive injury and repair resulting in hyperplasia[3]. Whereas acute injury and inflammation associated with healing are usually self-limited, chronic injury or inflammation leads to a sustained expansion of tissue proliferation[7-10]. Sustained tissue proliferation is generally accepted as a risk factor for cancer[3]. As is well known, metaplastic gastritis is precursors of gastric cancer.
Our understanding of gastritis and cancer underwent a marked shift with rediscovery of H pylori[7-13]. H pylori is now thought to account for most of gastritis[13] whereas H pylori infection is not an only important factor for development for gastric cancer[5]. But, it is not clear whether H pylori infection is also important for development for metaplastic gastritis. Our study demonstrated that H pylori seropositivity is an independent risk factor for the metaplastic gastritis.
In the intestinal type of gastric cancer, environmental factors other than H pylori infection seem to play a part in the carcinogenesis[12]. Environmental factors may facilitate the development of atrophic gastritis and intestinal metaplasia[12,14]. Based on epidemiologic studies of dietary histories, the first step in the Correa pathway was believed to be initiated by a diet rich in salt and nitrates/nitrites as well as deficiencies in fresh fruits and vegetables[5]. Dietary factors and continued effects of chronic inflammation were felt to be responsible for the progression from gastritis to atrophy, metaplasia, dysplasia and carcinoma[12]. Ingestion of sodium chloride is thought to promote gastric carcinogenesis[14]. Exposure to N-nitroso compounds probably facilitates advancement of chronic atrophic gastritis and intestinal metaplasia in adulthood[5]. Our study showed that neither dietary habits, salts nor folate intakes is a risk factor for the metaplastic gastritis. The major limitation of our study is that diet survey used in our study is carried out by not-validated questionnaire.
Both superficial and chronic atrophic gastritis are common in alcoholics[6]. Alcohol consumption can also cause acute gastritis[15]. Our study demonstrated that alcohol is not a risk factor for the metaplastic gastritis. Male dominance of the metaplastic gastritis can be explained, in which male gender tends to have more dangerous environmental factors such as smoking. But, our study demonstrated that male gender and smoking are independent risk factors for the metaplastic gastritis, respectively.
First, the limitation of present study is selection-bias. The substantial numbers of subjects did not have information about all items of the questionnaire. Numbers of subjects entered into the multiple logistic regression model were 4 438 (4 438/13 578, 32%) in group II and 3 483 (3 483/10 521, 33%) in group I. The other limitation of our study is inter-examiner or intra-examiner bias of endoscopic diagnosis about metaplastic gastritis and normal. To ascertain the precision in endoscopic diagnosis of gastritis, we undertook a pilot study on 10 individuals. Endoscopic finding was obtained 2 times on 10 individuals by 2 examiners. We examined the inter-examiner bias between 2 examiners and intra-examiner bias between 2 examinations by same examiner. Kappa value of intra-examiner was 1.0 (P = 0.002), 0.737 (P = 0.016), respectively. Kappa value of inter-examiner was 0.875 (P < 0.001).
Our data suggested that old age, male gender, H pylori seropositivity and smoking were risk factors for metaplastic gastritis, precancerous lesion of gastric cancer.
S- Editor Wang J L- Editor Zhang JZ E- Editor Bai SH
| 1. | 2002 Annual report of the Korea central cancer registry. Available form: URL: http: //www.yubang.com/down/2002cancer_regi_result.pdf. |
| 2. | Correa P. Chronic gastritis as a cancer precursor. Scand J Gastroenterol Suppl. 1984;104:131-136. [PubMed] |
| 3. | Houghton J, Wang TC. Helicobacter pylori and gastric cancer: a new paradigm for inflammation-associated epithelial cancers. Gastroenterology. 2005;128:1567-1578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 186] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 4. | Basso D, Stefani A, Brigato L, Navaglia F, Greco E, Zambon CF, Piva MG, Toma A, Di Mario F, Plebani M. Serum antibodies anti-H. pylori and anti-CagA: a comparison between four different assays. J Clin Lab Anal. 1999;13:194-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Yamaguchi N, Kakizoe T. Synergistic interaction between Helicobacter pylori gastritis and diet in gastric cancer. Lancet Oncol. 2001;2:88-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 63] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | You WC, Blot WJ, Chang YS, Ershow AG, Yang ZT, An Q, Henderson B, Xu GW, Fraumeni JF Jr, Wang TG. Diet and high risk of stomach cancer in Shandong, China. Cancer Res. 1988;48:3518-3523. [PubMed] |
| 7. | Zhang C, Yamada N, Wu YL, Wen M, Matsuhisa T, Matsukura N. Helicobacter pylori infection, glandular atrophy and intestinal metaplasia in superficial gastritis, gastric erosion, erosive gastritis, gastric ulcer and early gastric cancer. World J Gastroenterol. 2005;11:791-796. [PubMed] |
| 8. | Faraji EI, Frank BB. Multifocal atrophic gastritis and gastric carcinoma. Gastroenterol Clin North Am. 2002;31:499-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Sipponen P, Hyvärinen H, Seppälä K, Blaser MJ. Review article: Pathogenesis of the transformation from gastritis to malignancy. Aliment Pharmacol Ther. 1998;12 Suppl 1:61-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 66] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Meining A, Morgner A, Miehlke S, Bayerdörffer E, Stolte M. Atrophy-metaplasia-dysplasia-carcinoma sequence in the stomach: a reality or merely an hypothesis. Best Pract Res Clin Gastroenterol. 2001;15:983-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 59] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2805] [Cited by in RCA: 2739] [Article Influence: 80.6] [Reference Citation Analysis (0)] |
| 12. | Genta RM, Rugge M. Review article: pre-neoplastic states of the gastric mucosa--a practical approach for the perplexed clinician. Aliment Pharmacol Ther. 2001;15 Suppl 1:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Kuipers EJ, Siersema PD. The aetiology and clinical relevance of gastric intestinal metaplasia. Dig Liver Dis. 2004;36:501-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 14. | Montani A, Sasazuki S, Inoue M, Higuchi K, Arakawa T, Tsugane S. Food/nutrient intake and risk of atrophic gastritis among the Helicobacter pylori-infected population of northeastern Japan. Cancer Sci. 2003;94:372-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Bujanda L. The effects of alcohol consumption upon the gastrointestinal tract. Am J Gastroenterol. 2000;95:3374-3382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 204] [Article Influence: 8.2] [Reference Citation Analysis (0)] |