Published online Apr 21, 2006. doi: 10.3748/wjg.v12.i15.2375
Revised: October 12, 2005
Accepted: October 26, 2005
Published online: April 21, 2006
AIM: To study the expression and activity of inducible nitric oxide synthase (iNOS) and endothelial nitric oxide synthase (eNOS) in rats with ethanol-induced liver injury and their relation with liver damage, activation of nuclear factor-κB (NF-κB) and tumor necrosis factor-α (TNF-α) expression in the liver.
METHODS: Female Sprague-Dawley rats were given fish oil (0.5 mL) along with ethanol or isocaloric dextrose daily via gastrogavage for 4 or 6 wk. Liver injury was assessed using serum alanine aminotransferase (ALT) activity and pathological analysis. Liver malondialdehyde (MDA), nitric oxide contents, iNOS and eNOS activity were determined. NF-κB p65,iNOS, eNOS and TNF-α protein or mRNA expression in the liver were detected by immunohistochemistry or reverse transcriptase-polymerase chain reaction (RT-PCR).
RESULTS: Chronic ethanol gavage for 4 wk caused steatosis, inflammation and necrosis in the liver, and elevated serum ALT activity. Prolonged ethanol administration (6 wk) enhanced the liver damage. These responses were accompanied with increased lipid peroxidation, NO contents, iNOS activity and reduced eNOS activity. NF-κB p65, iNOS and TNF-α protein or mRNA expression were markedly induced after chronic ethanol gavage, whereas eNOS mRNA expression remained unchanged. The enhanced iNOS activity and expression were positively correlated with the liver damage, especially the necro-inflammation, activation of NF-κB, and TNF-α mRNA expression.
CONCLUSION: iNOS expression and activity are induced in the liver after chronic ethanol exposure in rats, which are correlated with the liver damage, especially the necro-inflammation, activation of NF-κB and TNF-α expression. eNOS activity is reduced, but its mRNA expression is not affected.
- Citation: Yuan GJ, Zhou XR, Gong ZJ, Zhang P, Sun XM, Zheng SH. Expression and activity of inducible nitric oxide synthase and endothelial nitric oxide synthase correlate with ethanol-induced liver injury. World J Gastroenterol 2006; 12(15): 2375-2381
- URL: https://www.wjgnet.com/1007-9327/full/v12/i15/2375.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i15.2375
Nitric oxide (NO) has been recognized as an important mediator of physiological and pathophysiological processes. It is produced by at least two isoforms of nitric oxide synthase (NOS) in the liver, such as eNOS and iNOS. eNOS is a Ca2+- and calmodulin-dependent constitutive isoform and plays an important role in vasorelaxation, whereas iNOS is not a constitutive enzyme and its expression may be induced by stimuli such as lipopolysaccharide or proinflammatory cytokines[1,2].
The role of NO in alcohol-induced liver injury still remains controversial. Nanji et al[3] reported that arginine, a substrate for NO, can significantly attenuate ethanol-induced liver injury. Treatment with N-nitro-L-arginine methyl ester (L-NAME), a nonselective NOS inhibitor, enhances alcohol-induced liver injury in the Tsukamoto-French enteral rat model[4]. However, iNOS knockout mice or wild-type mice treated with N-(3-aminomethyl) benzyl-acetamindine (1400W), a highly selective iNOS inhibitor, are protected against liver damage caused by alcohol[5]. Uzun et al[6] showed that L-NAME might produce a restorative effect on ethanol-induced liver damage.
Nuclear factor-KB (NF-KB) is a ubiquitous transcription factor that plays an important role in regulation of inflammatory responses. NF-KB is composed of homo- and hetero-dimers of five members of the Rel family, including NF-KB1 (p50), NF-KB2 (p52), Rel A (p65), Rel B, and Rel C. The most prevalent activated form of NF-KB is a heterodimer consisting of a p50 or a p52 subunit and p65. NF-KB exists in cytoplasm in an inactive form associated with regulatory proteins called IKB. After stimulation, it is translocated to the nuclei and bound to decameric DNA sequences, and activates transcription of target genes[7]. NF-KB has been shown to be functionally important for iNOS induction[8]. In the present study, we used fish oil plus ethanol gavage model of alcoholic liver disease, and examined the expression and activity of iNOS and eNOS in the liver, and their relation with liver damage, activation of NF-KB and TNF-α expression.
Polyclonal rabbit anti-iNOS and anti-NF-KB p65 were obtained from Santa Cruz Biotechnology, Inc. Biotinylated goat-anti-rabbit IgG was purchased from Beijing Zhongshan Reagent Corp. TRIzol reagent was purchased from Invitrogen. DL2000 DNA ladder marker was from TaKaRa Biotech Co., Ltd. M-MLV reverse transcriptase and its buffer, deoxyribonucleotide (dNTP, 10 mmol/L) and oligo(dT)15 primer were from Promega Corp. Taq DNA polymerase and its buffer, rRNasin ribonuclease inhibitor was from Biostar. Polymerase chain reaction (PCR) primers for eNOS, iNOS, TNF-α and GAPDH, were synthesized by Sai-Bai-Sheng Biocompany (Shanghai, China). Malondialdehyde (MDA), nitric oxide (NO) and nitric oxide synthase (NOS) activity assay kits were purchased from Nanjing Jiancheng Bioengineering Co.Ltd, China.
Female Sprague-Dawley rats weighing 200-250 g, were obtained from the Experimental Animal Center of Wuhan University. After acclimation for 6-7 d, animals were randomly divided into 4-wk dextrose group (n = 5), 6-wk dextrose group (n = 5), 4-wk ethanol group (n = 8), and 6-wk ethanol group (n = 8). Rats were given 0.5 mL fish oil along with ethanol or isocaloric dextrose intragastrically by gavage. The initial dose of ethanol was 6 g/kg per day (solutions maximally containing 56 mL/100 mL alcohol), and the dose was progressively increased during wk 1 to a maintenance dose of 8 g/kg per day that was continued for another 3 or 5 wk. All rats had free access to regular standard rat chow throughout the experiment. The animals were weighed three times per wk. At the end of the experiment, the animals were anaesthetized with urethane (20%, 1.0 g/kg) and sacrificed by bleeding from femoral arteries and veins. Blood samples were collected. Immediately after exsanguination, the livers were harvested. Small portions of the liver were kept at -70 °C for reverse transcriptase-polymerase chain reaction (RT-PCR) analysis, whereas another portion was separated and immersed in 10% buf-fered formalin solution for histological and immunohistochemical examination. All animals were given humane care in compliance with the institutional guidelines.
Liver specimens, 1.0 cm×0.5 cm×0.3 cm in size, were processed for light microscopy. This processing consisted of fixing the specimens in 10% formaldehyde for 12-24 h, embedding them in paraffin, slicing sections of 5 μm in thickness and staining the sections with hematoxylin and eosin. Histological assessment was performed by a pathologist unaware of the study. The severity of liver pathology was assessed as follows[9]: steatosis (the percentage of liver cells containing fat), 1+, <25% of cells containing fat; 2+, 26%-50% of cells containing fat; 3+, 51%-75% of cells containing fat; and 4+, >75% of cells containing fat. Necrosis was evaluated as the number of necrotic foci/mm2 and inflammation was scored as the number of inflammatory cells/mm2.
Blood samples were allowed to clot, and the sera were isolated by centrifugation at 1000 r/min for 10 min and kept at -20 °C before determination. Enzymatic activity of alanine aminotransferase (ALT) was measured using a commercial kit by an RA 1 000 automatic biochemical analyzer (Japan).
Liver samples were thawed, weighed and homogenized 1 : 9 w : v in 0.9% saline. Then the homogenates were centrifuged at 3 000 r/min for 10 min at 4 °C and the supernatant was taken for the assays of MDA contents, NOS activity and total protein.
MDA was assayed by measuring the thiobarbituric acid-reactive substances (TBARS) levels spectrophotometrically at 532 nm. Results were expressed as nmol.mg-1 protein.
NOS catalyzed the formation of NO and L-citrulline from L-arginine and molecular oxygen, and NO reacted with a nucleophile to generate color compounds. The absorbance at 530 nm NOS activity was calculated and expressed as U/mg protein. One unit of NOS activity was defined as the production of 1 nmol nitric oxide per second per mg tissue protein. Total NOS activity was measured as follows: 10% tissue homogenate (100 µL) was incubated with 200 µL substrate buffer, 10 µL reaction accelerator and 100 µL color development reagent at 37 °C for 15 min after mixing. Then 100 µL clearing reagent and 2 mL stop solution were added, mixed and absorbances were read at 530 nm. For measuring iNOS activity, an inhibitor was added before incubation according to the manufacturer's instructions.
Total protein concentration was determined using the Coomassie blue method with bovine serum albumin as standard.
Liver samples were thawed, weighed and homogenized 1 : 9 w : v in 0.9% saline. The homogenates were then centrifuged at 1 000 r/min for 5 min at 4 °C, the supernatant was taken for NO assay and total protein determination.
NO was assayed spectrophotometrically by measuring total nitrate plus nitrite (NO3- plus NO2-) and the stable end products of NO metabolism. In the procedure nitrate was enzymatically converted into nitrite by the enzyme nitrate reductase, followed by quantitation of nitrite using Griess reagent at the absorbance of 550 nm as previously described[10]. Results were expressed as μmol/g protein.
Five μm thick sections were prepared from paraffin-embedded tissues. After deparaffinization, endogenous peroxidase was quenched with 3% H2O2 in deionised water for 5-10 min. Nonspecific binding sites were blocked by incubating the sections in 10% normal rabbit serum for 10-15 min. The sections were then incubated with polyclonal rabbit anti-iNOS (dilution 1 : 25) or anti-NF-kB p65 (dilution 1 : 100) overnight at 4 °C, followed by incubation with biotinylated goat-anti-rabbit IgG at room temperature for 10-15 min. After 3×3 min PBS rinses, the horseradish-peroxidase-conjugated streptavidin solution was added and incubated at room temperature for 10-15 min. The antibody binding sites were visualized by incubation with a diaminobenzidine-H2O2 solution. The sections incubated with PBS instead of the primary antibody were used as negative controls. Brown-yellow granules in cytoplasm or nuclei were recognized as positive staining for iNOS or NF-kB p65 respectively. NF-kB immunoreactivity was expressed as the number of positive cells/high-power field (×400).
Total RNA was isolated from approximatively 50-100 mg snap-frozen liver tissue using the TRIzol protocol as suggested by the supplier. Following precipitation, the RNA was resuspended in RNAse-free buffer, the concentration was assayed by measuring ultra-violet light absorbance at 260 nm and purity was estimated from the ratio of A260/A280.
Single-stranded complementary DNA (cDNA) was synthesized from the total RNA using the following method. In brief, 2 μg RNA was preincubated with 0.5 μg oligo(dT)15 primer and diethylpyrocarbonate (DEPC)-treated water was added to a total volume of 15 μL at 70 °C for 5 min, then rapidly chilled on ice. To the annealed primer/template 5 µL M-MLV 5×reaction buffer, 1.25 µL dNTP (10 mmol/L, each), 25 units of rRNasin ribonuclease inhibitor, 200 units of M-MLV RT and DEPC-treated water were added to a final volume of 25 μL. The reaction was incubated at 42 °C for 60 min and terminated by placing it on ice after deactivation at 85 °C for 5 min. The resulting cDNA was used as a template for subsequent PCR.
The PCR mixture contained 5 µL of 10×Taq buffer, 1 µL of dNTP (10 mmol/L, each), 1 µL of gene specific primers (Table 1, sense and anti-sense primers, 25 pmol/μL, each), 2.0 units of Taq DNA polymerase and 1 µL of cDNA in a total volume of 50 μL. Thirty-five cycles of amplification were performed with initial incubation at 94 °C for 3 min and a final extension at 72 °C for 7 min, each cycle consisted of denaturation at 94 °C for 45 s, annealing at 54 °C for 45 s and extension at 72 °C for 1 min. To ensure the use of equal amounts of cDNA from each group samples in PCR, the aliquots of the reverse transcription products were used in PCR with the primers for house-keeping gene GAPDH. The quantities of cDNA producing equal amounts of GAPDH-PCR-product were used in PCR with the primers for iNOS, eNOS and TNF-α. Following RT-PCR, 5 μL samples of amplified products was resolved by electrophoresis on 2% agarose gel and stained with ethidium bromide. The level of each PCR product was semi-quantitatively evaluated using a di-gital camera and an image analysis system (Vilber Lourmat, France), and normalized to GAPDH.
| Name | Sense | Antisense | Product length (bp) |
| iNOS | TTCTTTGCTTCTGTGCTAATGCG | GTTGTTGCTGAACTTCCAATCGT | 1061 |
| eNOS | TGGGCAGCATCACCTACGATA | GGAACCACTCCTTTTGATCGAGTTAT | 202 |
| TNF-α | GCCAATGGCATGGATCTCAAAG | CAGAGCAATGACTCCAAAGT | 357 |
| GAPDH | TCCCTCAAGATTGTCAGCAA | AGATCCACAACGGATACATT | 309 |
Results were presented as mean ± SD unless otherwise indicated. Differences between groups were analyzed using analysis of variance with post hoc analysis using LSD test. The correlation was analyzed with Spearman’s correlation coefficients. P < 0.05 was considered statistically significant.
In each of the four groups, the rats increased their weight at a constant rate. There was no difference in weight gain among the groups.
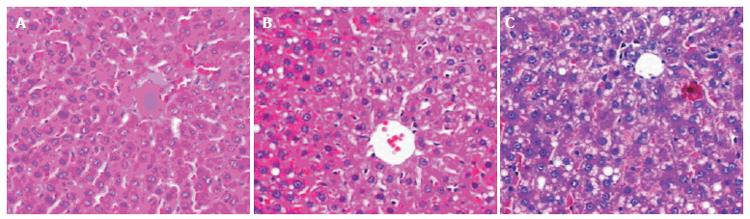
The animals given fish oil plus dextrose developed slight steatosis in the liver, but no obvious inflammation or necrosis was observed (Figure 1A). However, chronic ethanol gavage for 4 wk caused steatosis, minimal to mild inflammation and necrosis in the liver (Figure 1B). Prolonged ethanol administration (6 wk) enhanced the liver damage. Pronounced macrovesicular and microvesicular steatosis as well as spotty necrosis and mild inflammation were observed (Figure 1C, Table 2).
| Groups | Fatty liver | Necroinflammation | ALT(U/L-1) | MDA(nmol/mg-1 protein) |
| 4-wk dextrose | 0.2 ± 0.25 | 1.0 ± 1.0 | 43.4 ± 3.05 | 4.83 ± 0.70 |
| 4-wk ethanol | 2.1 ± 0.64a | 16.1 ± 2.17 a | 117.3 ± 6.92 a | 8.64 ± 0.62 a |
| 6-wk dextrose | 0.6 ± 0.35 | 1.6 ± 1.52 | 46.0 ± 3.16 | 5.36 ± 0.31 |
| 6-wk ethanol | 2.9 ± 0.64b | 19.9 ± 4.29 b | 124.1 ± 7.28 b | 9.38 ± 0.39 b |
Consistent with the histological changes, serum ALT levels, an index of liver cell injury, were significantly increased in 4-wk ethanol group, and further increased in 6-wk ethanol group as compared with dextrose groups (Table 2).
Liver contents of MDA, a marker of lipid peroxidation, were significantly increased after 4 wk ethanol gavage compared with dextrose groups. Prolonged ethanol exposure (6 wk) led to a further increase in MDA contents (Table 2).
Levels of NO in the liver of two dextrose groups were 0.75 ± 0.14 and 0.87 ± 0.07 μmol/g protein, respectively. Chronic ethanol gavage-induced NO level was two-fold higher in 4-wk ethanol group and further higher in 6-wk ethanol group (Table 3).
| Groups | NO (µmol/g-1 protein) | iNOS (U/mg-1 protein) | eNOS(U/mg-1 protein) |
| 4-wk dextrose | 0.75 ± 0.14 | 0.27 ± 0.07 | 0.64 ± 0.06 |
| 4-wk ethanol | 1.67 ± 0.15 | 0.60 ± 0.07 a | 0.48 ± 0.03 a |
| 6-wk dextrose | 0.87 ± 0.07 | 0.35 ± 0.06 | 0.58 ± 0.04 |
| 6-wk ethanol | 1.84 ± 0.12 b | 0.70 ± 0.09 b | 0.43 ± 0.05 b |
The isoforms of NOS present in the liver were mainly eNOS and iNOS as previously reported[2]. The amount of total NOS activity minus iNOS activity might represent the activity of eNOS. Chronic fish oil plus ethanol gavage led to a marked elevation in iNOS activity with further elevation in 6-wk ethanol group compared with 4-wk ethanol group. In contrast, the eNOS activity was significantly reduced compared with dextrose groups (Table 3).
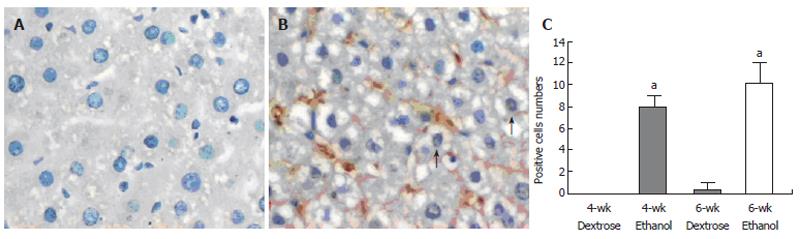
NF-KB p65 staining was present in cytoplasm and nuclei, but only nuclear staining was considered positive. There was no positive staining in two dextrose groups, whereas following chronic ethanol administration, remarkable enhancement in the positive staining was observed (Figures 2A and 2B). The number of positive cells in 4-wk and 6-wk ethanol groups was 8.0 ± 1.1 and 10.0 ± 1.9 /high-power field, respectively (Figure 2C). The NF-KB p65 positive cells were primarily Kupffer cells and hepatocytes.
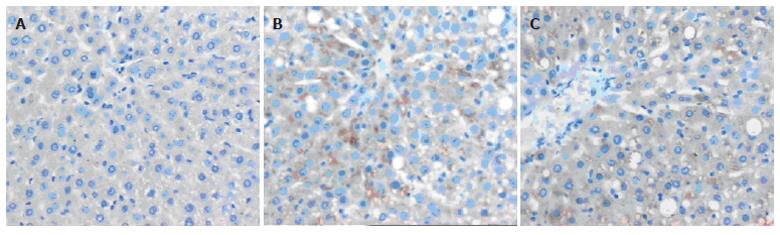
Only faint immunoreactive staining of iNOS was detected in the liver from dextrose groups (Figure 3A). However, intense staining of iNOS was observed in 4-wk ethanol group (Figure 3B), and more intense staining was found in 6-wk ethanol group (Figure 3C). The staining was mainly present in both severely damaged and perivascular areas.
iNOS mRNA was barely detectable in the liver of dextrose groups, but after chronic ethanol gavage (4 wk), iNOS mRNA was markedly induced. Prolonged ethanol gavage (6 wk) was associated with more intense bands (Figure 4). In dextrose groups only faint bands for TNF-α were detected. After chronic ethanol gavage, similar expression pattern of iNOS was also observed in TNF-α mRNA expression (Figure 4).
In contrast, there was no significant change in eNOS mRNA expression between dextrose and ethanol groups (Figure 4).
Correlation analysis showed that liver iNOS activity was positively correlated with the severity of liver damage (steatosis, necroinflammation) (r = 0.71 and 0.93, respectively, P < 0.05), especially the necroinflammation. iNOS expression was only detected in rats with liver damage, activation of NF-KB and intense TNF-α mRNA expression. The intensity of the former paralleled that of the latter.
It has been reported that dietary fatty acids play an important role in the pathogenesis of alcoholic liver disease[11,12]. Polyunsaturated fatty acids enriched in fish oil promote alcoholic liver injury and pathological changes occur only in rats fed with ethanol containing polyunsaturated fatty acids[13]. Alcoholic liver injury is more severe and develops rapidly in women than in men[14]. Our study employed female rats and used fish oil plus ethanol gavage to make an animal model of alcoholic liver injury. The rats developed pathological changes in the liver after 4 or 6 wk, such as steatosis, spotty necrosis and inflammation, all of which resemble alterations found in clinical alcoholic liver disease. This chronic gavage of alcohol in rats is a simple experimental model that mimics key aspects of alcoholic liver disease in humans, and is useful for exploring the mechanism and treatment of alcoholic liver disease.
NO is an important biological mediator and has been shown to be involved in diverse physiological as well as pathological processes[1]. In our study, chronic ethanol gavage led to a significant elevation of liver NO contents compared to dextrose groups. NO is generated by NOS. We assayed the activity of total NOS and iNOS in liver. Because the main isoforms of NOS in the liver are eNOS and iNOS[2], the amount of total NOS activity minus iNOS activity may represent the activity of eNOS. Our study showed that iNOS activity was significantly elevated after chronic ethanol consumption in rats, whereas eNOS activity was markedly reduced as compared with dextrose groups. Accompanying the enhanced activity, iNOS expression detected by immunohistochemistry and RT-PCR in the liver was significantly increased in ethanol groups compared to dextrose groups. However, the eNOS mRNA expression was comparable between these groups. The results suggest that the elevated NO release in the liver is attributable to the enhanced activity and expression of iNOS. Relationship analysis showed that enhanced iNOS activity and expression were associated with the severity of liver damage, especially the necroinflammation, suggesting that iNOS contributes to alcohol-induced liver injury. It was reported that iNOS knockout mice or wild-type mice treated with 1400W, a highly selective iNOS inhibitor, are protected against alcohol-induced liver injury[5]. In contrast with iNOS, eNOS activity is reduced after treatment with ethanol. A recent report showed that chronic alcohol intake attenuates hepatic eNOS activity by increasing the expression of the inhibitory protein caveolin-1 and enhancing its binding to eNOS[15]. Treatment with L-NAME, the stronger eNOS inhibitor, exacerbates alcohol-induced liver injury[4]. These facts suggest that the role of NO in ethanol-induced liver injury may be dependent on the isoforms of NOS.
Oxidant stress has been reported to play a role in the pathogenesis of alcohol-induced liver injury[16]. In this study, liver contents of MDA, a marker of lipid peroxidation, were significantly elevated in the ethanol groups as compared with dextrose groups. Oxidant stress can result in degradation of the cytoplasmic NF-KB inhibitor, IKB, allowing translocation of NF-KB to nuclei[17]. Our study showed that chronic ethanol gavage significantly enhanced the expression of active NF-KB in the liver, as evidenced by the increased number of NF-KB p65 positively stained (in nuclei) cells. Furthermore, TNF-α mRNA expression in the liver was markedly increased. These results are consistent with the report of Nanji et al[18], who showed that NF-KB is activated during alcoholic liver disease in the presence of pro-inflammatory stimuli, resulting in increased expression of pro-inflammatory cytokines and chemokines. In our study, correlation analysis showed that iNOS expression was positively associated with NF-KB p65 expression, suggesting that increased iNOS expression may be caused by the activation of NF-KB. Activated NF-KB, a transcription factor, may bind to the specific DNA sequence of iNOS to promote its expression[19].
In conclusion, iNOS expression and activity induced in ethanol-induced liver injury are responsible for the elevated NO production. The induction of iNOS is associated with liver damage, especially necroinflammation, activation of NF-KB and elevated TNF-α mRNA expression in the liver.
S- Editor Wang J L- Editor Wang XL E- Editor Ma WH
| 1. | Chen T, Zamora R, Zuckerbraun B, Billiar TR. Role of nitric oxide in liver injury. Curr Mol Med. 2003;3:519-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 124] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 2. | McNaughton L, Puttagunta L, Martinez-Cuesta MA, Kneteman N, Mayers I, Moqbel R, Hamid Q, Radomski MW. Distribution of nitric oxide synthase in normal and cirrhotic human liver. Proc Natl Acad Sci U S A. 2002;99:17161-17166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 141] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 3. | Nanji AA, Jokelainen K, Lau GK, Rahemtulla A, Tipoe GL, Polavarapu R, Lalani EN. Arginine reverses ethanol-induced inflammatory and fibrotic changes in liver despite continued ethanol administration. J Pharmacol Exp Ther. 2001;299:832-839. [PubMed] |
| 4. | Nanji AA, Greenberg SS, Tahan SR, Fogt F, Loscalzo J, Sadrzadeh SM, Xie J, Stamler JS. Nitric oxide production in experimental alcoholic liver disease in the rat: role in protection from injury. Gastroenterology. 1995;109:899-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 78] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | McKim SE, Gäbele E, Isayama F, Lambert JC, Tucker LM, Wheeler MD, Connor HD, Mason RP, Doll MA, Hein DW. Inducible nitric oxide synthase is required in alcohol-induced liver injury: studies with knockout mice. Gastroenterology. 2003;125:1834-1844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 196] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 6. | Uzun H, Simsek G, Aydin S, Unal E, Karter Y, Yelmen NK, Vehid S, Curgunlu A, Kaya S. Potential effects of L-NAME on alcohol-induced oxidative stress. World J Gastroenterol. 2005;11:600-604. [PubMed] |
| 7. | Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001;107:7-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3011] [Cited by in RCA: 3175] [Article Influence: 132.3] [Reference Citation Analysis (0)] |
| 8. | Taylor BS, Alarcon LH, Billiar TR. Inducible nitric oxide synthase in the liver: regulation and function. Biochemistry (Mosc). 1998;63:766-781. [PubMed] |
| 9. | Nanji AA, Jokelainen K, Tipoe GL, Rahemtulla A, Thomas P, Dannenberg AJ. Curcumin prevents alcohol-induced liver disease in rats by inhibiting the expression of NF-kappa B-dependent genes. Am J Physiol Gastrointest Liver Physiol. 2003;284:G321-G327. [PubMed] |
| 10. | Tarpey MM, Wink DA, Grisham MB. Methods for detection of reactive metabolites of oxygen and nitrogen: in vitro and in vivo considerations. Am J Physiol Regul Integr Comp Physiol. 2004;286:R431-R444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 437] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 11. | Nanji AA. Role of different dietary fatty acids in the pathogenesis of experimental alcoholic liver disease. Alcohol. 2004;34:21-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Purohit V, Russo D, Coates PM. Role of fatty liver, dietary fatty acid supplements, and obesity in the progression of alcoholic liver disease: introduction and summary of the symposium. Alcohol. 2004;34:3-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Nanji AA, Zhao S, Sadrzadeh SM, Dannenberg AJ, Tahan SR, Waxman DJ. Markedly enhanced cytochrome P450 2E1 induction and lipid peroxidation is associated with severe liver injury in fish oil-ethanol-fed rats. Alcohol Clin Exp Res. 1994;18:1280-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 191] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 14. | Ashley MJ, Olin JS, le Riche WH, Kornaczewski A, Schmidt W, Rankin JG. Morbidity in alcoholics. Evidence for accelerated development of physical disease in women. Arch Intern Med. 1977;137:883-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 103] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Wang X, Abdel-Rahman AA. Effect of chronic ethanol administration on hepatic eNOS activity and its association with caveolin-1 and calmodulin in female rats. Am J Physiol Gastrointest Liver Physiol. 2005;289:G579-G585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Arteel GE. Oxidants and antioxidants in alcohol-induced liver disease. Gastroenterology. 2003;124:778-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 362] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 17. | Asehnoune K, Strassheim D, Mitra S, Kim JY, Abraham E. Involvement of reactive oxygen species in Toll-like receptor 4-dependent activation of NF-kappa B. J Immunol. 2004;172:2522-2529. [PubMed] |
| 18. | Nanji AA, Jokelainen K, Rahemtulla A, Miao L, Fogt F, Matsumoto H, Tahan SR, Su GL. Activation of nuclear factor kappa B and cytokine imbalance in experimental alcoholic liver disease in the rat. Hepatology. 1999;30:934-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 181] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 19. | Spink J, Cohen J, Evans TJ. The cytokine responsive vascular smooth muscle cell enhancer of inducible nitric oxide synthase. Activation by nuclear factor-kappa B. J Biol Chem. 1995;270:29541-29547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 121] [Article Influence: 4.0] [Reference Citation Analysis (0)] |