INTRODUCTION
The colon is the end part of the gastrointestinal tract from the ileocecal valve to the anal region. For many years, barium examination and endoscopy were the only proven diagnostic methods for evaluating diseases of the colon. In the last 15-20 years, however, spiral computed tomography (CT) has also been shown to be an essential tool in radiological evaluation of the gastrointestinal tract. CT and MRI can be used to evaluate diseases of the colon. They can show the colon wall, colon lumen and the adjacent tissues and organs. CT and MRI are particularly useful in the initial staging of colon neoplasms, assessing the response of colon tumors to therapy and postoperative recurrence of gastrointestinal tumors, evaluating possible causes for gastrointestinal organ displacement and extrinsic impressions detected by barium studies or endoscopy. Moreover, CT and MRI are useful for the detection of inflammatory bowel disease[1-5]. Selective three-dimensional (3D) imaging of the colon was first described in 1994 by Vinning et al[6] as a method using spiral CT to provide a computer-simulated endoluminal perspective of the air distended colon. In 1997, Luboldt et al[8] first described 3D imaging of the colon filled with paramagnetic contrast as MR colonography[6-8].
Colorectal cancer is the third most common cancer and the second leading cause of cancer-related death in western countries. Most colorectal cancer evolves from pre-existing adenomatous polyps. The incidence of colorectal cancer could be considerably reduced if polyps and small tumors were detected and eliminated prior to their malignant degeneration[9-11]. There is ongoing research for a colorectal cancer screening test that is cost-effective, safe, and acceptable to patients. Current screening methods for colorectal polyps and colonic cancer include fecal occult blood testing, sigmoidoscopy, colonoscopy and double contrast barium enema examination. The effectiveness of each modality as a screening tool remains controversial, and each method has inherent limitations. MR colonography based on MR imaging is a relatively new diagnostic modality for diagnosing colon pathology.
The purpose of this prospective study was to evaluate the sensitivity and specificity of MR colonography and CT in 42 patients who were suspected of having colonic lesions. Standard colonoscopy and histopathologic examination were accepted as the reference standard.
MATERIALS AND METHODS
Patients
A total of 42 patients (25 men, 17 women; mean age 59.3, range 2-85 years), who were suspected of having colonic lesions because of rectal bleeding, positive fecal occult blood test results or altered bowel habits, underwent MR colonography and CT examination, followed by a conventional colonoscopy (CC). All patients underwent standard bowel preparation 24 h before examination. All patients gave written informed consent, and the procedures were approved by the Local Ethics Committee.
Methods
MR colonography was performed on a 1.5T MR system (Edge, Picker, USA). No sedative or analgesic agents were used. Patients were placed in a supine position on the MR table. After insertion of a rectal tube, the colon was filled with 1000-1500 mL of a mixture of 0.9 g/L NaCl solution (1000 mL) and 0.5 mmol/L gadopentetate dimeglumine (15-20 mL) and 300 g/mL iodinized contrast material (100 mL). When the contrast material reached the cecum, the 3D colon imaging data were acquired using a T1W 3D Gradient-Echo Sequence (GRE) (TE: 2.49 ms, TR: 6 ms, flip angle: 10, thickness: 2.5 mm, FOV: 40 cm-43 cm, matrix: 128 × 192). Further sequences were performed on all patients of axial spin-echo (SE) T1W (TE: 10 ms, TR: 130 ms, flip angle: 90, thickness: 8 mm, FOV: 40 cm-43 cm matrix: 192 × 256), and fatsat SE T1W (TE: 20 ms, TR: 749 ms, flip angle: 90, thickness: 7 mm, FOV: 40 cm-43 cm, matrix: 192 × 256).
Immediately after the MR colonography, abdominal CT images were taken on the spiral CT (PQS, Picker, USA) in the axial planes ( kV: 130, mA: 175, thickness: 5 mm, matrix: 512 × 512) and supine position. The three-dimensional MR data sets were analyzed in the multiplanar reformation and evaluated completely and separately and independently by two experienced radiologists. The CT axial plane images were evaluated by both the experienced radiologists. Each radiologist recorded the location and the size of colorectal masses or defined the colon lumen, wall and adjacent tissues to lesions of the large bowel, respectively. If their interpretation of the MR colonography and CT images differed, consensus was reached by review and discussion of the controversial images.
All patients were examined with the same endoscopist video (Pentax EC 38 40 TL, Tokyo, Japan) colonoscope. The location and size of any endoluminal lesions were identified and the colon wall pathologic appearance was recorded. All lesions found by CC were biopsied or removed by polypectomy. All specimens were examined histologically for differential diagnosis of inflammatory disease, hyperplastic polyps, adenomatous polyps, and cancers.
Standard colonoscopy and histopathologic examination were accepted as the references, so the MR colonography and CT were being evaluated for sensitivity, specificity and correct diagnosis ratio in the detection of colonic lesions. Each MRC examination lasted about 20-30 min, CT examination about 10-15 min and CC examination about 20-30 min. MR colonography and CT were well tolerated by all patients with no post-procedural complications after MR colonography, CT or CC.
RESULTS
A total of 42 patients suspected of having colonic lesions underwent MR colonography and abdominal spiral CT. Colonic lesions were identified by MR colonography in 26 patients. MR colonography was normal in 16 patients. On the basis of MR colonography and CT, 17 colon carcinoma (65.3%), 2 invasion to rectum (7.7%), 1 recurrent colon tumor (3.8%), 4 inflammatory bowel disease (15.3%), 1 hirschsprung disease (3.8%), and 1 diverticulosis (3.8%) were determined and these lesions were confirmed by conventional colonoscopy and histopathologic examination.
MR colonography and CT identified colorectal cancer in 17 patients. Malignant tumors of the colon were located in the rectum (6), rectosigmoid region (3), caecum (2), ascending colon (1) (Figure 1), descending colon (1) and sigmoid colon (4) (Figure 2). Malignant tumors of the colon appeared on MR colonography and CT as a tumor mass projecting into the lumen of the colon or as an asymmetrical or circumferential thickening of the bowel wall with deformation and narrowing of the lumen. Fourteen adenocarcinoma, two mucinous adenocarcinoma and one tubulovillous adenoma (carcinoma in situ) were confirmed by histopathologic examination. Seventeen patients with colon tumors underwent CC. A complete CC was achieved in 14 patients. In three patients, CC could not be evaluated completely due to occlusive carcinoma. However, in these patients, all of the colon segments were examined by MR colonography. The results of the MR colonography and CT were compared with the colonoscopy, histopathologic examination and surgery results. All patients with colon tumors had been correctly identified on MR colonography and CT.
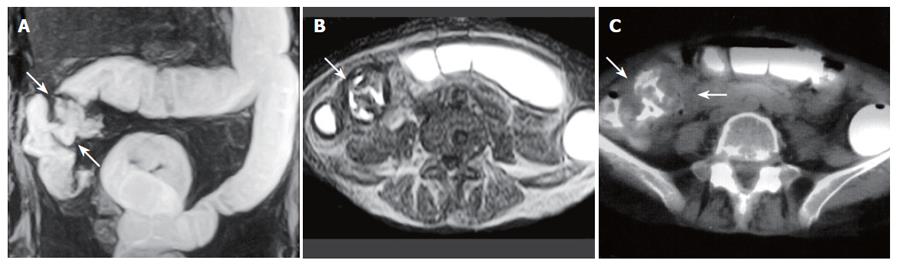
Figure 1 A 59-year-old woman with cecum and ascending colon carcinoma.
A: MR colonography MIP (maximum intensity projection) image showing asymmetrical, irregular wall thickening at the cecum and ascending colon segments (arrows); B: SE T1W axial image; C: axial CT image showing asymmetrical, irregular wall thickening of the ascending colon (arrows).
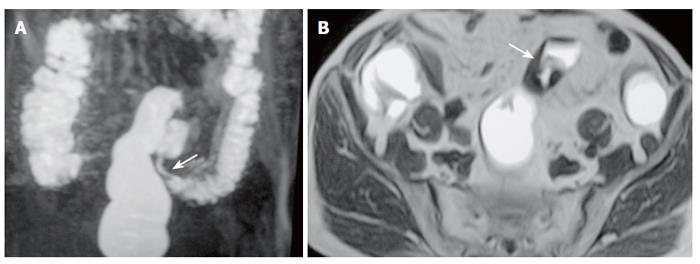
Figure 2 A 77-year-old man with sigmoid carcinoma.
A: MR colonography MIP (maximum intensity projection) image showing asymmetrical, annular wall thickening at the sigmoid colon segment (arrow); B: SE T1W axial image showing asymmetrical, irregular wall thickening of the sigmoid colon (arrow).
Four patients with inflammatory bowel disease were correctly identified on MR colonography and CT. These were identified by histopathologic examination as ulcerative colitis (Figure 3), non-specific colitis and ileocaecal region inflammatory disease (Figure 4). In one patient non-specific colitis was evaluated by histopathologic examination and colonoscopy, but it was not identified by MR colonography and CT. Thus MRC and CT failed to identify 20% (1/5) patients with inflammatory colon disease. In 2 patients with colon carcinoma, the colonoscopy detected polyps (size: 3 mm in one patient and 7 mm in the other) which had not been diagnosed with MR colonography. In one of them, the MR examination had been insufficient because of technical reasons. In the other patient, the small polyp was not identified from the adjacent mass. In the 2 patients with colon carcinoma and one patient with ulcerative colitis, colonoscopy detected polyps (size: 3 mm in 1 patient, 5 mm in 1 patient and 7 mm in the other) which had not been diagnosed with CT. In all three cases, the CT examination had been insufficient because of technical reasons and the small polyps had not been identified from the adjacent mass.
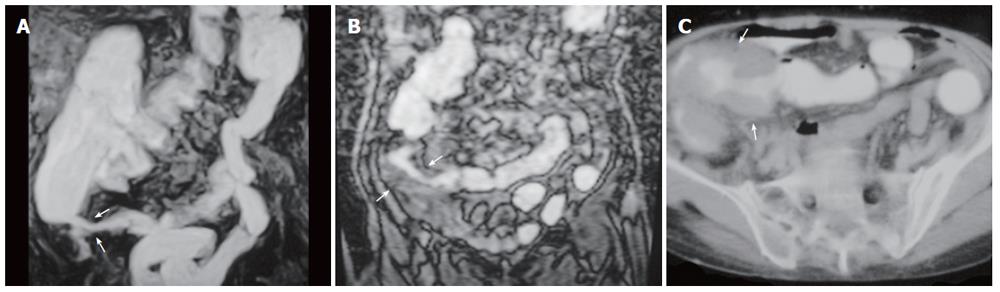
Figure 3 A 63-year-old man with ulcerative colitis.
A: MR colonography MIP (maximum intensity projection) image; B: axial CT image showing haustral flattening at transverse and descending colon segments (arrows); C: SE T1W axial image showing a 0.5-cm polyp at the rectosigmoid junction (arrow).
Figure 4 A 70-year-old man with inflammatory disease in the ileocaecal region.
A: MR colonography MIP (maximum intensity projection) image; B: coronal plane MR colonography raw data image; C: axial CT image showing asymmetrical, irregular wall thickening of the ileocaecal region (arrows) and serpiginous strands extending into soft tissue due to inflammatory disease.
MR colonography and CT identified invasion to the rectum in two patients. In one case where there was bladder cancer invasion to the rectum, both the MR colonography and CT examinations identified invasion to the rectum and further MR colonography showed the presence of rectovesical fistula due to bladder cancer. In the other patient, an invasion to the rectum due to prostate cancer was detected (Figure 5). The sensitivity and specificity of MR colonography for colon pathologies were 96.4% and 100%, respectively. The percentage of correct diagnosis of MR colonography was 97.6%. The sensitivity and specificity of CT for colon pathologies were 92.8% and 100%, respectively. The rate of correct diagnosis of CT was 95.2%. The MR colonography was well tolerated without sedation or analgesia and no complications were observed. MR colonography and CT identified 19 extracolonic lesions in 12 of 42 patients. These lesions were liver metastases, hydatic cyst of the liver, simple cyst of the liver, mesenteric cyst, gallbladder carcinoma, duodenum carcinoma, renal cyst, gastric tumor, multiple lymphadenopathy, intraperitoneal lipoma, hiatal hernia, subcapsular hematoma of the spleen and pleural effusion.
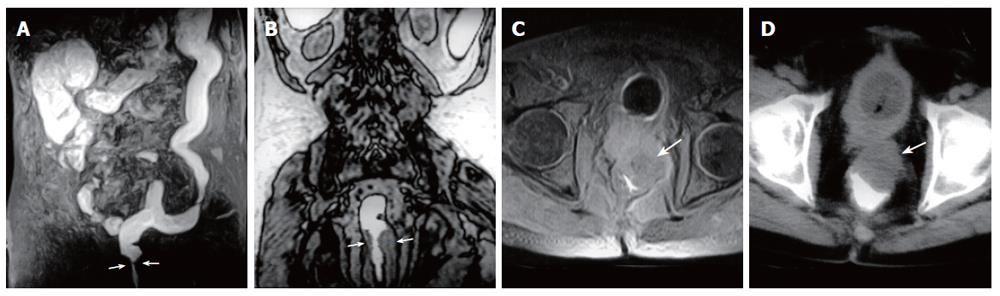
Figure 5 A 63-year-old man with rectum carcinoma invasion to prostate.
A: MR colonography MIP (maximum intensity projection) image showing luminal narrowing at rectum (arrows); B: MR colonography raw data coronal image showing luminal narrowing due to annular thickening of the rectum wall (arrows); C: SE axial T1W image; D: axial CT image showing asymmetric wall thickening at rectum (arrows), and invasion to the fatty tissue arround the mass.
DISCUSSION
Colorectal cancer is the second leading cause of cancer-related death. Most colorectal cancer evolves from adenomatous polyps and screening for colorectal polyps with subsequent polypectomy has been shown to constitute an effective approach to decreasing its incidence[9,10]. However, as evidenced by disappointing participation in colorectal screening and the continuing high incidence of colorectal cancer, new screening strategies may prove beneficial. To prove effective in reducing mortality from colorectal cancer, new screening methods must demonstrate a high diagnostic accuracy at a low cost, and be proven safe and highly acceptable to patients[12].
Fecal occult blood testing has a sensitivity of less than 10% for adenomatous polyps, and a sensitivity of less than 15% for the detection of polyps under 2 cm in size[13]. In contrast, the promise of MR colonography is to detect malignant and premalignant polyps with a sensitivity rivaling colonoscopy.
Flexible sigmoidoscopy allows for the examination of only the distal 60 cm of the colon which limits evolution to the descending colon, sigmoid, and rectum; inevitably, lesions are missed in more than half the subjects who have advanced colonic adenoma located proximal to the splenic flexure but who do not have a distal index polyp[14,15]. Colonography, in contrast, images the entire colorectum and may be able to decrease the mortality by detecting more right-sided lesions. In retrospective evaluations of double-contrast barium enema examination, investigators have found sensitivities of 71%-95% for the detection of colorectal cancer[16]. However, in prospective studies of double-contrast barium enema examination, data have shown the sensitivities as low as 50%-75% for colorectal cancer detection in asymptomatic patients with positive fecal occult blood test[17]. A recent study in which double-contrast barium enema examination was compared with colonoscopy for colonic surveillance after polypectomy found a poor detection rate of 48% for polyps ≥ 10 mm in size, as well as a poor overall detection rate of only 39% for adenomas[18]. Unlike barium enema, MR colonography does not suffer from superimposition and can explain the attenuation characteristics of suspicious lesions, as well as allowing for the evaluation of pericolonic tissues.
In most centers, colonoscopy has emerged as the principal means of examining the colon. Although standard colonoscopy is a total colonic examination that allows lesion biopsy and resection, it fails to demonstrate the entire colon in up to 5% of cases examined by an experienced gastroenterologist[19], and up to 20% of all adenomas are missed[20].
Furthermore, there is a risk of complications associated with diagnostic and therapeutic colonoscopy, including perforation (1 in 1000), major haemorrhage (3 in 1000), and death (1 in 30 000)[12,20]. In addition, colonoscopy is limited by poor patient acceptance, which is a most important variable for a screening test[21,22]. Rex et al[23] have shown that even when it is offered free of charge, most patients refused to undergo colonoscopy for primary colorectal cancer screening.
In colorectal cancer screening, MR colonography can play an important role for patients who have undergone incomplete endoscopic colonoscopy. Common reasons for incomplete colonoscopy are redundant bowel loops and occlusive carcinoma. MR colonography can achieve a complete examination of the colon in these patients. In patients with occlusive carcinoma, the evaluation of the proximal colon is necessary to exclude a secondary neoplasia, which occurs in 5% of these cases[24]. MR colonography is available for detecting colorectal masses. Luboldt et al[25] performed MR colonography in 132 patients referred for CC, showing a sensitivity of 93% and specificity of 99% of MR colonography. A similar study by Pappalardo et al[26] compared MR colonography with conventional colonoscopy in 70 patients. All patients who underwent MR colonography had satisfactory studies and MR colonography achieved a diagnostic accuracy similar to that of conventional colonoscopy (sensitivity of 96%, specificity of 93%).
MR colonography techniques have recently been introduced as potential methods for colorectal screening. MR colonography may have a role in accurately staging colorectal cancers, in particular if combined with state-of-the-art MR imaging of the liver. In the same manner as for staging, MR colonography can also be used for post-operative surveillance[27]. Besides the detection and assessment of neoplastic disease, MR colonography can also be employed for the evaluation of inflammatory bowel disease. Over the past decade, several authors have found that MR imaging is a useful, non-invasive tool in patients with Crohn’s disease, regardless of whether it is manifested in the small bowel, large bowel, or in the perianal region[28-30]. MR imaging can be used to assess disease activity and may help distinguish reversible inflammatory changes from irreversible fibrostenosis[31-33]. In contrast to double-contrast barium enema or conventional colonoscopy, MR colonography might be used to distinguish the Crohn’s strictures that require surgery from those that might benefit from anti-inflammatory therapy, in addition to visualizing the colon proximal to a narrowing, and assessing extracolonic complications of the disease, including fistula and abscesses[34].
An enema with dilute iodinated contrast material can be administered well by the rectal tube in advance of the CT study, just prior to imaging. CT can also be used to evaluate the colon wall. In its characterization of colon neoplasms, CT is useful to categorize the extent of tumor as follows: intraluminal mass without wall thickening; wall thickening focal or diffuse with no extramural tumor extension; invasion of contiguous mesenchymal tissue; invasion of adjacent organs or other anatomic structures; involvement of regional lymph nodes; or metastatic spread to distant organs, lymph nodes or other structures[1-3]. CT colonography is available for detecting colorectal masses. Pickhardt et al[35] performed CT colonography in 1233 asymptomatic adults referred for CC, and found the sensitivity of virtual colonoscopy for adenomatous polyps was 93.8% for polyps at least 10 mm in diameter, 93.9% for polyps at least 8 mm in diameter, and 88.7% for polyps at least 6 mm in diameter. The specificity of virtual colonoscopy for adenomatous polyps was 96.0% for polyps at least 10 mm in diameter, 92.2% for polyps at least 8 mm in diameter, and 79.6% for polyps at least 6 mm in diameter. CT virtual colonoscopy with the use of a three-dimensional approach is an accurate screening method for the detection of colorectal neoplasia in asymptomatic average-risk adults and compares favorably with optical colonoscopy in terms of the detection of clinically relevant lesions[35].
CT can show diverticulosis and inflammatory bowel disease. CT findings in inflammatory disease include circumferential wall thickening, serpiginous soft-tissue strands extending into the mesenteric fat, and enlarged lymph nodes in the same region[5].
MR colonography and CT were used on 5 patients with inflammatory bowel disease, and 4 of 5 were correctly identified. MR colonography and CT failed to correctly identify in one patient who was diagnosed to have non-specific colitis by histopathologic examination. Currently, colonoscopy is generally reserved for patients with positive results from screening tests or those with a higher than average-risk of colorectal cancer, rather than applying it for routine screening. In the search for an adequate screening method, MR colonography has emerged relatively strongly. MR colonography possesses unique advantages over existing screening tests in that it is quick, less invasive, with no need for sedation or analgesics during investigation and with a lower percentage of perforation complications. Moreover, it enables evaluation of all colon segments because of multi-sectional imaging availability, thus enabling to evaluate intramural, extra-intestinal components of colonic lesions, metastasis and additional lesions. MR colonography is a fundamentally new imaging technique with the potential to alter current clinical approaches in the detection of colorectal neoplasms and inflammatory bowel disease.
In conclusion, in the search for a rapid, less invasive, accurate, and well-tolerated colorectal examination method, magnetic resonance colonography can be an effective method.