Published online Apr 21, 2006. doi: 10.3748/wjg.v12.i15.2341
Revised: October 9, 2005
Accepted: October 10, 2005
Published online: April 21, 2006
AIM: To investigate the selective cytotoxic effect of constructed hybrid protein on cells expressing granulocyte macrophage colony stimulating factor (GM-CSF) receptor.
METHODS: HepG2 (human hepatoma) and LS174T (colon carcinoma) were used in this study. The fused gene was induced with 0.02 % of arabinose for 4 h and the expressed protein was detected by Western blotting. The chimeric protein expressed in E.coli was checked for its cytotoxic activity on these cells and apoptosis was measured by comet assay and nuclear staining.
RESULTS: The chimeric protein was found to be cytotoxic to the colon cancer cell line expressing GM-CSFRs, but not to HepG2 lacking these receptors. Maximum activity was observed at the concentration of 40 ng/mL after 24 h incubation. The IC50 was 20 ± 3.5 ng/mL.
CONCLUSION: Selective cytotoxic effect of the hybrid protein on the colon cancer cell line expressing GM-CSF receptors (GM-CSFRs) receptor and apoptosis can be observed in this cell line. The hybrid protein can be considered as a therapeutic agent.
- Citation: Roudkenar MH, Bouzari S, Kuwahara Y, Roushandeh AM, Oloomi M, Fukumoto M. Recombinant hybrid protein, Shiga toxin and granulocyte macrophage colony stimulating factor effectively induce apoptosis of colon cancer cells. World J Gastroenterol 2006; 12(15): 2341-2344
- URL: https://www.wjgnet.com/1007-9327/full/v12/i15/2341.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i15.2341
Although systemic treatment of colon cancer including combination of surgery, hormone therapy, chemotherapy is commonly believed to be effective in prolonging patient survival, colon cancer remains one of the principal cancer-related deaths[1]. Human cancer is becoming more definable by cell surface proteins. One of these molecules is GM-CSF receptor that expresses in several tumors inclu-ding colon cancer[2].
GM-CSF is used to attenuate the myelosupressive effects of chemotherapy in the treatment of hematologic malignancies and solid tumors, but GM-CSF usually does not stimulate growth of solid tumors[2]. It was reported that GM-CSFR is absent on most immature hematopoietic progenitors but increases expression during maturation[3]. This finding strongly suggests that GM-CSFR may be a useful target for recombinant toxins or immunotoxins.
Immunotoxin comprises cells targeting and killing moieties. Therefore, bacterial or plant toxins are used as the killing moieties. Shiga and Shiga-like toxins (STX, SLTs) are ribosome-inactivating proteins (RIPs) produced by Shigella and E.coli, composed of an enzymatic A subunit non-covalently associated with a pentamer receptor-binding subunit. SLTs inhibit protein synthesis in eukaryotic cells by releasing adenine residue from the highly conserved aminoacyl-tRNA-binding site which exists on large subunit of ribosome [4]. In this study, the catalytic domain of Shiga toxin (StxA1) and hGM-CSF were genetically fused and expressed in E.coli. Then, the function of recombinant protein was assessed on colon cancer cell line.
The catalytic domain of Shiga toxin, StxA1, was fused to hGM-CSF by PCR technique and expressed in bacteria with 0.02 % arabinose for 4 h incubation. The expressed protein was purified following the manufacturer's instructions (Invitrogen, USA). The amount of protein was estimated by Bio-Rad protein assay kit (Bio-Rad, USA).
HepG2 cell line derived from human hepatoma and LS174T cell line derived from colon carcinoma were provided by Cell Bank of Institute of Development, Aging and Cancer, Tohoku University, Japan. These cell lines were kept in RPMI-1640 medium (Gibco-BRL, Germany) supplemented with 10 % fetal bovine serum (Gibco-BRL , Germany ) at 37 °C in 50 mL /L CO2 atmosphere.
The cytotoxic effect of SltxA1-GM-CSF was determined by trypan blue exclusion assay and MTT[5] assay. For trypan blue dye exclusion assay, 2.5 × 104 cells were seeded in one well of 96-well plates and different concentration (10-160 ng) of SltxA1-GM-CSF was added to each well. After incubation for 24, 48, and 72 h, the number of viable cells was determined.
For MTT assay, HepG2 and LS174T cell lines were cultured and StlxA1-GM-CSF was added as described above. After 24, 48 or 72 h incubation, 10 µL of 0.5 mg/mL of 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma, Japan) was added to each well and further incubated at 37 °C in 50 mL/L CO2 atmosphere for 4 h to allow MTT to be converted to formazon crystals by reacting with metabolically active cells. Reactions were stopped by addition of 10 % SDS in 0.01 mol/L HCl and absorbance at 570 nm was determined.
Neutral comet assay was carried out as previously des-cribed[6] with some modifications. Briefly, freshly prepared cell suspension (2 × 103 / 10 µL) was mixed with 150 µL of 1% low-melting agars. The mixture was layered on top of the microscopic slide coated by 1 % agarose. After low melting agarose was solidified in a refrigerator for 10 min, the slide was gently immersed in a freshly prepared lysing solution (2 % SDS, 0.03 mol/L EDTA) for 30 min protected from light. After the slides were washed with TBE buffer, electrophoresis was carried out at 25 V for 25 min. Then, comets were visualized with 1 mmol/L propodium iodide. One hundred and fifty cells per slide were analysed under fluorescent microscope.
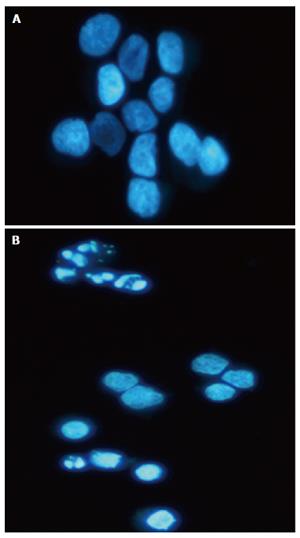
After administration of SltxA1-GM-CSF, cells were fixed with 1 % paraformaldehyde for 30 min, washed with PBS and stained with 1mmol/L Hoechst 33342 (Sigma, Japan) for 10 min. Nuclear morphology of at least 400 cells was randomly observed under fluorescent microscope.
The results were expressed as mean ± SD and each value represented the mean of three experiments. Differences between groups were compared using the Student’s t test. P < 0.05 was taken as significant.
The fusion protein consisting of 1-255 a.a. of A1, the catalytic domain of Shiga-toxin and 1-127 a.a. of hGM-CSF was observed. The recombinant was analysed by SDS-PAGE and Western blot as previously described[7].
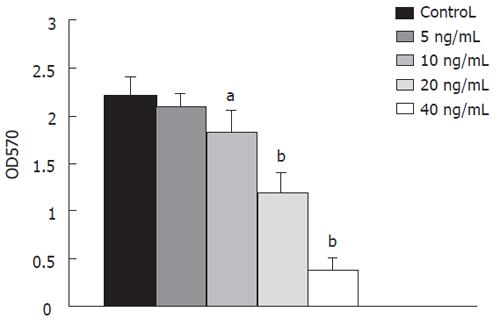
Cytotoxicity of hGM-CSF to cells was analysed by both trypan blue exclusion assay and MTT assay. LS174T cells showed the extreme susceptibility to StxA1-GM-CSF (Figure 1), while HepG2 cells lacking GM-CSFR showed very poor susceptibility to StxA1-GM-CSF. Maximum activity was observed at the concentration of 40 ng/mL (P < 0.001) after 24 h incubation. Higher concentration and longer treatment time did not show any profound effect. The IC50 was 20 ± 3.5 ng/mL.
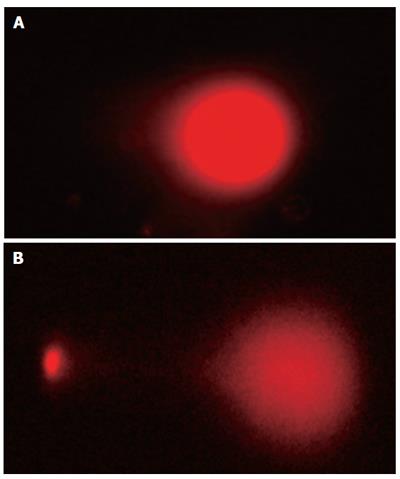
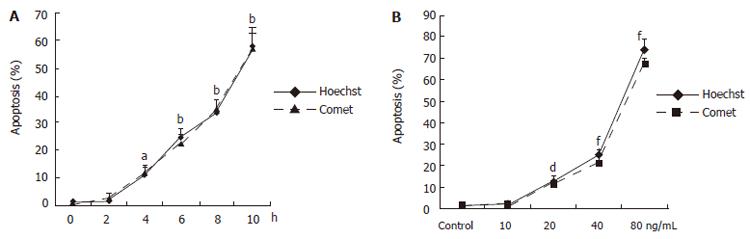
We next examined death of LS174T cells induced by the StxA1-GM-CSF by comet assay. In comet assay apoptotic cells after treatment showed puffy tails and pin heads while normal cells had spherical heads but no tails (Figure 2). To verify the apoptotic results obtained by comet assay, cells were analyzed by Hoechst 33342 staining. Apoptotic cells with nuclear condensation and fragmentation were analyzed (Figure 3). The results indicated that the apoptotic cells were effectively induced by the toxin (Figure 4).
In this study we constructed the recombinant protein of StxA1-GM-CSF by overlap extension PCR, which was expressed in pBAD/gIIIA system[7]. Al-Jaufy et al[8] have also made the recombinant protein genetically. They fused StxA with HIV gp120-binding domain of CD4 by restriction enzyme sites. However, some limiting factors like incomplete digestion, absence of suitable restriction sites for genes of interest and appropriate vector have limited the application of this method. Accuracy and ease of manipulation, are likely the advantages of PCR technique.
StxA is an established inhibitor of eukaryotic translation and more potent than ricin[9]. No reduction in its enzyma-tic inhibitory activity has been reported when it is genetically fused with HIV gp120-binding domain of CD4[8]. Similarly in our study, fusion of StlxA1 with hGM-CSF caused no reduction in its inhibitory effect. In addition, the enzymatic domain of diphtheria toxin has been fused to hGM-CSF using recombinant DNA techniques and is under clinical trial[10]. Our results suggest that StxA1-GM-CSF is more toxic than DT-GM-CSF, since IC50 is 20 ± 3.5 ng/mL for StxA1-GMCSF and 70 ± 18 ng/mL for DT-GM-CSF[8]. In this study, LS174T showed very obvious cytotoxic effect and changes characteristic of apoptosis were observed. A close correlation between protein synthesis inhibition and apoptosis induction has also been reported for DT-GM-CSF in which LS174T is not sensitive to apoptosis while other cell lines are more sensitive to cytotoxicity and apoptosis[11].
Agarose gel electrophoresis is one of the commonly used techniques for the detection of apoptotic cells. This technique usually involves a DNA isolation procedure from millions of cells and obtained results can not be quantified[12]. TUNEL assay is an established method for the detection of apoptotic cells, but this method is associated with a number of artefacts[13]. Annexin V labelled with fluorescent protein like FITC is used for rapid cytofluorometric analysis of apoptosis, but this method can produce false positive results when membrane is damaged[14]. Comet assay is sensitive, simple and fast. Usually this method is used to detect DNA damage like single or double strand breaks. On the other hand, this method also can detect single apoptotic cells in a large number of cells[15]. A few studies have tried to delineate the mechanism of apoptosis induction by Stx[16-18]. More knowledge about its mechanism can help us prevent shigellosis and enable us to tackle the problem of resistance to cancer therapy. Induction of apoptosis by hybrid protein indicates that catalytic domain of Shiga toxin in hybrid protein can not activate the cellular apoptosis machinery directly since the toxin inactivates ribosomes that inhibit protein synthesis.
In conclusion, more investigations are required to clarify how StxA1-GMCSF induces apoptosis. Our results reveal that the hybrid protein is toxic to the colon cancer cell line and can be considered as a therapeutic agent.
The authors thank Li Li for his technical assistance.
S- Editor Wang J L- Editor Wang XL E- Editor Wu M
| 1. | Rougier P, Mitry E. Epidemiology, treatment and chemoprevention in colorectal cancer. Ann Oncol. 2003;14 Suppl 2:ii3-ii5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Kreitman RJ, Pastan I. Recombinant toxins containing human granulocyte-macrophage colony-stimulating factor and either pseudomonas exotoxin or diphtheria toxin kill gastrointestinal cancer and leukemia cells. Blood. 1997;90:252-259. [PubMed] |
| 3. | Wognum AW, Westerman Y, Visser TP, Wagemaker G. Distribution of receptors for granulocyte-macrophage colony-stimulating factor on immature CD34+ bone marrow cells, differentiating monomyeloid progenitors, and mature blood cell subsets. Blood. 1994;84:764-774. [PubMed] |
| 4. | Stirpe F. Ribosome-inactivating proteins. Toxicon. 2004;44:371-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 231] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 5. | Tada H, Shiho O, Kuroshima K, Koyama M, Tsukamoto K. An improved colorimetric assay for interleukin 2. J Immunol Methods. 1986;93:157-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 635] [Cited by in RCA: 657] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 6. | Olive PL, Wlodek D, Banáth JP. DNA double-strand breaks measured in individual cells subjected to gel electrophoresis. Cancer Res. 1991;51:4671-4676. [PubMed] |
| 7. | Habibi Roudkenar M, Bouzari S, Oloomi M, Jafari A, Shahrokhi N, Shokrgozar MA. Expression of a chimeric protein containing the catalytic domain of Shiga-like toxin and human granulocyte-macrophage colony-stimulating factor (hGM-CSF) in Escherichia coli and its recognition by reciprocal antibodies. IBJ. 2005;9:143-148 RA. |
| 8. | al-Jaufy AY, Haddad JE, King SR, McPhee RA, Jackson MP. Cytotoxicity of a shiga toxin A subunit-CD4 fusion protein to human immunodeficiency virus-infected cells. Infect Immun. 1994;62:956-960. [PubMed] |
| 9. | Skinner LM, Jackson MP. Inhibition of prokaryotic translation by the Shiga toxin enzymatic subunit. Microb Pathog. 1998;24:117-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Frankel AE, Powell BL, Hall PD, Case LD, Kreitman RJ. Phase I trial of a novel diphtheria toxin/granulocyte macrophage colony-stimulating factor fusion protein (DT388GMCSF) for refractory or relapsed acute myeloid leukemia. Clin Cancer Res. 2002;8:1004-1013. [PubMed] |
| 11. | Frankel AE, Hall PD, Burbage C, Vesely J, Willingham M, Bhalla K, Kreitman RJ. Modulation of the apoptotic response of human myeloid leukemia cells to a diphtheria toxin granulocyte-macrophage colony-stimulating factor fusion protein. Blood. 1997;90:3654-3661. [PubMed] |
| 12. | Wyllie AH, Kerr JF, Currie AR. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4980] [Cited by in RCA: 4834] [Article Influence: 107.4] [Reference Citation Analysis (0)] |
| 13. | Kockx MM, Muhring J, Knaapen MW, de Meyer GR. RNA synthesis and splicing interferes with DNA in situ end labeling techniques used to detect apoptosis. Am J Pathol. 1998;152:885-888. [PubMed] |
| 14. | Koester SK, Bolton WE. Differentiation and assessment of cell death. Clin Chem Lab Med. 1999;37:311-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Singh NP. A simple method for accurate estimation of apoptotic cells. Exp Cell Res. 2000;256:328-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 137] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 16. | Fujii J, Matsui T, Heatherly DP, Schlegel KH, Lobo PI, Yutsudo T, Ciraolo GM, Morris RE, Obrig T. Rapid apoptosis induced by Shiga toxin in HeLa cells. Infect Immun. 2003;71:2724-2735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 78] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Lee SY, Cherla RP, Caliskan I, Tesh VL. Shiga toxin 1 induces apoptosis in the human myelogenous leukemia cell line THP-1 by a caspase-8-dependent, tumor necrosis factor receptor-independent mechanism. Infect Immun. 2005;73:5115-5126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Cherla RP, Lee SY, Tesh VL. Shiga toxins and apoptosis. FEMS Microbiol Lett. 2003;228:159-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |