INTRODUCTION
Bombesin is originally isolated from Bombina bombina, a European frog. Utilizing antibodies to amphibian bombesin, bombesin-like immunoreactivity has been identified in mammalian brain, lung, and gut[1]. Bombesin has a wide range of biological effects that include release of hormones, stimulation of pancreatic enzyme secretion, inhibition of gastric emptying, modulation of gastric acid secretion and contractions of various smooth muscle preparations from isolated gut[2]. Until now, four different subtypes of bombesin receptors have been discovered, which can lead to the activation of multiple cell signaling pathways[3]. The primary structures of all these receptor subtypes as deduced from corresponding cDNAs, display seven transmembrane domains coupled to signaling pathways via heterotrimeric G proteins[4-6].
Protein kinase C (PKC) is a family of homologous serine/threonine kinases and presents in cytoplasm. Upon agonist stimulation, it rapidly translocates to the particulate or membrane fraction[7,8]. PKC plays a role in the regulation of sustained agonist-induced contraction of various vascular smooth muscle preparations[9].
Increased protein tyrosine phosphorylation occurs rapidly (within seconds) in response to both polypeptide growth factors and vasoconstrictor hormones[10,11]. Furthermore, epidermal growth factor and platelet-derived growth factor increase vascular tone[12], and are blocked by genistein and typhostins, both of which are inhibitors of tyrosine kinases, indicating that tyrosine phosphorylation is involved in the contractile response[12].
The p44/42 MAP Kinase pathway consists of a protein kinase cascade linking growth and differentiation signals with transcription in nuclei. Activated p44/p42 MAP kinase translocates to the nuclei and activates transcription by phosphorylation of transcription factors such as Elk-1 and Stat3. A selective and potent inhibitor of the p44/42 MAP kinase cascade, PD98059, has been identified[13]. This compound binds to inactive MEK and prevents phosphorylation and activation by Raf.
To test whether bombesin-induced contraction is mediated via a PKC- or PTK- or MAPK- dependent pathway and which G protein and phospholipase C isozyme is coupled to bombesin, we investigated the signals in mediating contraction induced by bombesin in cat esophageal circular muscle cells.
MATERIALS AND METHODS
Materials
R59949, PD98059 and SB202190 were purchased from Calbiochem (La jolla, CA). G protein antibodies (Gi1, Gi2, Gi3, Gq, and Go), and PLC isozyme antibodies (β1, β3, γ1) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). HEPES, collagenase type F, and other reagents were purchased from Sigma (St Louis, MO).
Preparation of dispersed muscle cells
Single muscle cells were isolated as previously described[14,15]. Muscle strip was incubated overnight in normal potassium HEPES buffer containing 1 g/L papain, 1 mmol/L DTT, 1 g/L bovine serum albumin (BSA) and 0.5 g/L collagenase (type F, Sigma) and equilibrated with 950 mL/L O2 and 5 mL/L CO2 to maintain a pH of 7.0 at 31 °C. The normal potassium-HEPES buffer contained 1 mmol/L CaCl2, 250 μmol/L EDTA, 10 mmol/L glucose, 10 mmol/L HEPES, 4 mmol/L KCl, 131 mmol/L NaCl, 1 mmol/L MgCl2 and 10 mmol/L taurine. Next day we warmed up the tissue at room temperature for 30 min and incubated the tissue in water bath at 31 °C for 30 min. After incubation, the digested tissue was washed with 50 mL enzyme-free solution on 360 μm Nitex filter and reincubated in enzyme-free solution at 31 °C and gassed with 950 mL/L O2 and 5 mL/L CO2 and the cells were allowed to disperse spontaneously for 10-20 min. Suspensions of single muscle cells were harvested by filtration through 500 μm Nitex meshes. Before beginning the experiment, the cells were heated at 31 °C for at least 10 min to relax the cells. Throughout the entire procedure, care was taken not to agitate the fluid to avoid cell contraction in response to mechanical stress.
Preparation of permeabilized smooth muscle cells
Cells were permeabilized to diffuse the agents such as G protein antibodies or PLC isozyme antibodies. After completion of the enzymatic phase of the digestion process, the partly digested muscle tissue was washed with an enzyme-free cytosolic buffer containing 20 mmol/L NaCl, 100 mmol/L KCl, 5.0 mmol/L MgSO4, 0.96 mmol/L NaH2PO4, 1.0 mmol/L EGTA, 0.48 mmol/L CaCl2, and 2% bovine serum albumin. The cytosolic buffer was equilibrated with 950 mL/L O2 and 5 mL/L CO2 to maintain a pH of 7.2 at 31 °C. Muscle cells dispersed spontaneously in this medium. The cytosolic buffer contained 0.48 mmol/L CaCl2 and 1 mmol/L EGTA, yielding 0.18 μmol/L free Ca2+ calculated as previously described[16]. After dispersion, the cells were permeabilized by incubation for 5 min in cytosolic buffer containing saponin (75 mg/L). After exposure to saponin, the cell suspension was centrifuged at 350 r/min and the resulting pellet was washed with saponin-free modified cytosolic buffer containing antimycin A (10 μmol/L), ATP (1.5 mmol/L) and an ATP-regenerating system consisting of creatine phosphate (5 mmol/L) and creatine phosphokinase (166.7μkat /L). After the cells were washed free of saponin, they were resuspended in modified cytosolic buffer.
Measurement of contraction by scanning micrometry
Contraction of isolated muscle cells was measured by scanning micrometry[17]. An aliquot of cell suspension containing 107 muscle cells/L was added to HEPES medium containing the test agents. The reaction was terminated by addition of formalin (10% final concentration). The length of 40-50 muscle cells treated with a contractile agent was measured at random by scanning micrometry, phase contrast microscope (model ULWCD 0.30 Olympus, Japan) and digital closed-circuit video camera (CCD color camera, Toshiba, Japan) connected to a Macintosh computer (Apple, Cupertino, CA) with a software program, NIH Image 1.57 (National Institutes of Health, Bethesda, MD) and compared with the length of untreated cells. Contraction was expressed as the percentage decrease in mean cell length from control.
Statistical analysis
Data were expressed as mean ± SE. Data differences between means were determined by Student's t test.
RESULTS
Characterization of G protein subtype-coupled receptor of bombesin
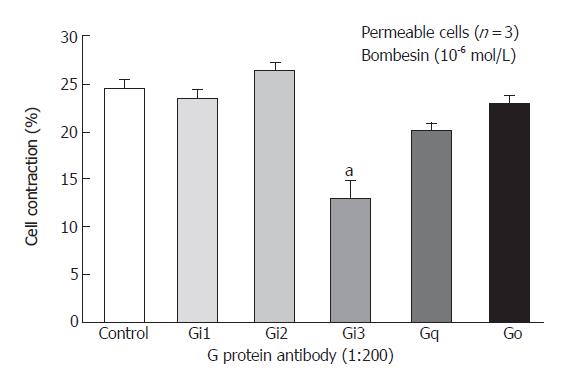
Freshly isolated smooth muscle cells were stimulated for 30 s with bombesin (10-6mol/L) and bombesin induced the contraction of smooth muscle cell (24.3% ± 2.2% decrease in cell length from control). G proteins, Gi1, Gi2, Gi3, G (40 ku), Gq (42 ku), Gs (46 ku) in cat esophagus cells were established as previously described[17,18]. To identify the specific G protein involved in cat esophagus contraction, muscle cells were permeabilized with saponin preincubated in cytosolic medium containing G protein antibody to allow the antibodies to diffuse into the cytosolic region of the cell membrane. These antibodies could block receptor-induced activation of G protein by binding to the terminal peptide region of G protein that could interact with the receptor. After permeabilization, the Gi3 inhibited contraction, but Gi1, Gi2, Go, Gq did not (Figure 1).
Figure 1 Inhibition of bombesin induced-contraction in permeabilized esophageal circular muscle cells by antibodies to G protein isoforms (Mean ± SE, Student’s t test, aP < 0.
05 vs Control).
PLC-β3 mediated bombesin-induced contraction
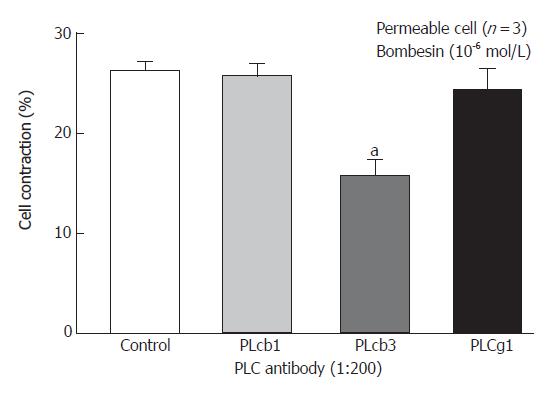
We have previously shown that PC-PLC inhibitor D609 could block the bombesin-induced contraction[17] and Western blot analysis of homogenates obtained from dispersed smooth muscle cells using monoclonal antibodies to PLC isozymes could demonstrate the presence of immunoreactive protein bands corresponding to 150 Ku PLC-β1, and PLC-β3 antibody, and 145 Ku PLC-β1 antibody[18]. Incubation of permeabilized circular muscle cells for 1 h with PLC- 3-specific antibody inhibited bombesin- (10-6 mol/L) induced contraction (P < 0.05). No other PLC-β3-specific antibodies had any significant effect on the contraction (Figure 2).
Figure 2 Inhibition of bombesin-induced contraction in permeabilized esophageal circular muscle cells by antibodies to PLC isoforms (Mean ± SE, Student’s t test, aP < 0.
05 vs Control).
Role of protein kinase C and tyrosine kinase in bombesin-induced contraction
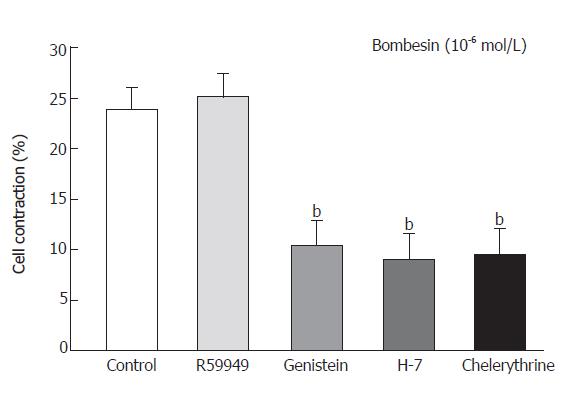
Cells were preincubated with either the tyrosine kinase inhibitor genistein (10-5 mol/L) for 20 min or with the protein kinase C inhibitor H-7 for 15 min (10-5 mol/L) or chelerythrine (10-5 mol/L) and diacylglycerol (DAG) kinase inhibitor R59949 (10-5 mol/L) for 1 min respectively, before the addition of bombesin (10-6mol/L). Bombesin-induced contraction was inhibited by preincubation with genistein as follows: percent age decrease in cell length was 24.3% ± 2.2% vs 10.4% ± 2.5% and 9.1% ± 2.5% vs 9.5% ± 2.5% in the cells preincubated with H-7 and chelerythrine, respectively (Figure 3).
Figure 3 Contractile response of smooth muscle cells from cat esophagus to bombesin in presence of protein kinase C inhibitors (mean ± SE, n = 4, Student’s t test, bP < 0.
01 vs Control).
Role of MAPK in bombesin-induced smooth muscle cells contraction
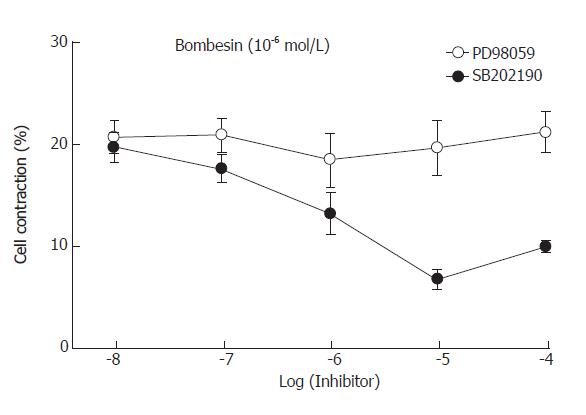
To examine which MAPK was involved in bombesin-induced contraction, specific MAPK inhibitors were used. Preincubation of PD98059 (the MEK inhibitor) blocked the contraction induced by bombesin in a concentration-dependent manner. The maximal inhibition was observed in 10-5mol/L (Figure 4). However, preincubation of SB202190 (the p38MAPK inhibitor) did not inhibit bombesin-induced concentration, suggesting that bombesin-induced concentration might be mediated via the p44/p42 MAPK pathway.
Figure 4 Effect of MEK inhibitor, PD98059 and p38 MAP kinase inhibitor, SB202190 on the bombesin-induced cat esophageal smooth muscle cell contraction (mean ± SE, n = 4).
DISCUSSION
Bombesin is an amidated tetradecapeptide originally purified from the skin of the European frog Bombina bombina[1]. Exogenous introduction of this peptide into various organ systems elicits a wide range of responses including secretion of gastrointestinal, adrenal and pituitary hormones and gastric acid, pancreatic enzyme and mucous, as well as regulation of smooth muscle contraction and modulation of neuronal firing rate[19]. Bombesin receptor is a member of the rhodopsin family of receptors containing seven transmembrane domains coupled to a G protein[20]. Molecular cloning studies have revealed the identity of three mammalian bombesin receptors: gastrin-releasing peptide (GRP receptor)[6], neuromedin B (NMB receptor)[21] and bombesin receptor subtype 3 (BRS-3)[4]. These three receptors share about 50% amino acid sequence identity. In adult animals, GRP-R and NMB-R are widely expressed in the central nervous system[21] and gastrointestinal tract[22,23], whereas BRS-3 shows limited expression in the hypothalamus but is expressed in secondary spermatocytes[4]. Peripheral administration of bombesin produces a variety of effects directly or indirectly linked to the activation of receptors for bombesin-like peptides in the gastrointestinal tract, including exocrine pancreatic secretion, gastrointestinal peptide hormone release, smooth muscle contraction and reduction of food intake. Bombesin receptor is activated after ligand binding and then catalyzes the exchange of GDP bound to the Gα subunit for GTP. After dissociation from Gβγ subunits, functional GTP binds to Gα subunit and activates β isoform of phospholipase C that catalyzes the hydrolysis of phosphatidyl inositol 4,5-bisphosphate (PIP2) in the cell membrane[24]. Gq (42 Ku), Gi1 (40 Ku), Gi2 (40 Ku), Gi3 (40 Ku), Go (40 Ku), Gs (46 Ku) in esophageal circular muscle have been detected by western blot[17]. In addition, when the same G protein antibodies are used to examine which G protein could mediate the contractile response of esophageal muscle, bombesin-induced contraction of esophageal muscles is inhibited by antibodies against the alpha subunits of Gi3[17].
We have previously shown that the response of esophageal cells to bombesin is blocked by the inhibitor of specific phosphatidylcholine-phospholipase C (PLC), D609[17] and Western blot analysis can demonstrate the presence of immunoreactive protein bands corresponding to 150 Ku PLC-β1, PLC-β3 antibody, and 145 Ku PLC-γ1 antibody[18]. Permeabilization was used to examine the participation of PLC isozymes in bombesin-induced muscle contraction in this study. Antibodies to PLC-β3, similarly to rabbit intestine[25], could inhibit bombesin-induced contraction. PLC-β1 and PLC-γ1 antibodies had no effect by themselves. The result suggests that PLC-β3 plays a role in mediating esophageal muscle contraction.
Many vasoconstrictor agonists increase protein tyrosine phosphorylation and ERK activity in smooth muscle preparations[26,27]. Furthermore, tyrosine kinase inhibitors block agonist-induced contraction[26,28,29], suggesting that this pathway is important for smooth muscle contraction. In this study, we investigated the regulation of tyrosine phosphorylation following bombesin stimulation and the role of this pathway in the contractile response. Genistein, a tyrosine kinase inhibitor, reduced contraction in response to bombesin in cat esophagus cells, suggesting that tyrosine kinases are involved in bombesin-induced contraction pathway.
Protein kinase C (PKC) is an enzyme activated by DAG, a second messenger produced by the PLC-catalyzed hydrolysis of PIP2. PIP2 hydrolysis produces two signaling molecules, DAG and IP3. DAG is the physiological activator of the classical and novel isoforms of PKC[30], whereas IP3 regulates intracellular Ca2+ movements[31]. DAG kinase plays an essential role in attenuation of DAG signals in agonist-stimulated cells. DAG kinase, which phosphorylates DAG to phosphatidic acid, is divided into a membrane bound and a soluble form. DAG kinase inhibitor increases PKC activity by blocking the phosphorylation of DAG to phosphatidic acid[32]. In this study, we showed that R59949 (the DAG kinase inhibitor) did not increase the bombesin-induced contraction. We found that PKC inhibitor, H-7 and chelerythrine, blocked the contraction induced by bombesin. This result is similar to those of Bitar et al[33].
MAP kinase is a serine/threonine-specific protein kinase, activation and phosphorylation of which are induced by a variety of extracellular factors such as mitogenic growth factors and neuropeptides[34,35]. In colonic smooth muscle cells[36,37], agonist-induced contraction involves a kinase cascade initiated by PKC and activation and redistribution of MAP kinase. The activation of MAP kinase by bombesin is rapid (within 15 s), reaching a maximum within 30 s, followed by a decline[37]. We found that p44/p42 MAP Kinase was involved in the bombesin-induced contraction in cat esophagus, indicating that the bombesin-induced signaling pathway progresses to p44/p42 MAP kinase to induce contraction.
In conclusion, bombesin induces circular muscle cell contraction in cat esophagus, which is mediated by protein kinase C and tyrosine kinase pathway. Bombesin induces contraction via Gi3 and PLC-β3. The contraction is mediated via p44/p42 MAP kinase pathway. Our findings provide the basic and clinical experimental data on bombesin-induced contraction and its signal transduction in esophagus.