Published online Apr 14, 2006. doi: 10.3748/wjg.v12.i14.2243
Revised: October 1, 2005
Accepted: December 22, 2005
Published online: April 14, 2006
AIM: To assess the actigraphy, an ambulatory and continuous monitoring of wrist motor activity fitted to study sleep/wake patterns in hepatic encephalopathy (HE).
METHODS: Twenty-five cirrhotic patients (17 M, 8 F, mean age 56 ± 11 years, 24/25 alcoholic, Child-Pugh A , B, C: 2, 6, 17) were included. The patients were classified into 3 groups: stage 0 group (n = 12), stage 1-2 group (n = 6), and stage 3-4 group (n = 7) of encephalopathy. Over three consecutive days, patients had clinical evaluation 3 times a day with psychometric test, venous ammoniemia, flash visually evoked potentials (VEP), electroencephalogram and continuous actigraphic monitoring for 3 d, providing 5 parameters: mesor, amplitude, acrophase, mean duration of activity (MDAI) and inactivity (MDII) intervals.
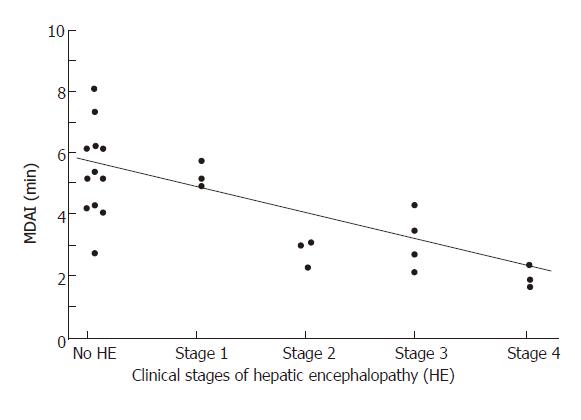
RESULTS: Serum ammonia and VEP did not differ among the 3 groups. Electroencephalography mean dominant frequency (MDF) correlated significantly with clinical stages of HE (r = 0.65, P = 0.003). The best correlation with HE stage was provided by actigraphy especially with MDAI (r = 0.7, P<10-4) and mesor (r = 0.65, P < 10-4). MDAI correlated significantly with MDF (r = 0.62, 0.004) and was significantly shorter in case of HE compared to patients without HE (stage 0: 5.33 ± 1.6 min; stage 1-2: 3.28 ± 1.4 min; stage 3-4: 2.52 ± 1.1 min; P < 0.05). Using a threshold of MDAI of less than 4.9 min, sensitivity, specificity, positive predictive value, negative predictive value for HE diagnosis were 85%, 67%, 73% and 80%, respectively.
CONCLUSION: Actigraphy may be an objective method to identify HE, especially for early HE detection. Motor activity at the wrist correlates well with clinical stages of HE. MDAI and mesor are the most relevant parameters.
- Citation: Hourmand-Ollivier I, Piquet MA, Toudic JP, Denise P, Dao T. Actigraphy: A new diagnostic tool for hepatic encephalopathy. World J Gastroenterol 2006; 12(14): 2243-2244
- URL: https://www.wjgnet.com/1007-9327/full/v12/i14/2243.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i14.2243
Hepatic encephalopathy (HE) diagnosis is based on clinical criteria. Serum ammonia, evoked potentials and electroencephalogram (EEG) have low sensitivity[1-3]. Actigraphy allows ambulatory and continuous monitoring of motor activity and is fitted to study sleep/wake patterns. The actigraph worn on the non dominant wrist is based on a miniaturized acceleration sensor that translates physical motion to a numeric representation. It has already been successfully used in insomnia and extra pyramidal syndrome[4,5]. We assessed the actigraphy in HE.
Twenty-five cirrhotic patients (17 M, 8 F, mean age 56 ± 11 years, 24/25 alcoholic, Child-Pugh A ,B, C: 2, 6, 17) were consecutively included. Patients with serum sedative or alcohol detection were excluded. Patients were classified into 3 groups: stage 0 group (n = 12), stage 1-2 group (n = 6), and stage 3-4 group (n = 7) of acute encephalopathy. HE causes were alcoholic hepatitis (n = 5), severe liver insufficiency (n = 3), digestive bleeding (n = 1), bacterial infection (n = 1), or undetermined (n = 3). Over three consecutive days, patients had clinical evaluation 3 times a day with the psychometric test Trail making test A when permitted by consciousness, venous ammoniemia using da Fonseca-Wollheim method, flash visual evoked potentials (VEP) with measurement of latencies of wavex N2, P2, N3, EEG and continuous actigraphic monitoring for 3 d using a small non dominant wrist-worn piezoelectric accelerometer (Gaehwiler electronic actimeter, CH 8634 Hombrechtiken, Switzerland) with a 0.1 G lower limit of sensitivity. Activity (wrist movements) measured at one-min intervals over a period of 24 h, was stored into the actigraph memory. Data downloaded to a compatible computer, were analysed by MONITOR software. The cosinor method or entire data linear adjustment by least-square method, provided 3 parameters: mesor (rhythm-adjusted mean), amplitude (half the variability between peaks to troughs), acrophase (peak time). Mean duration of activity (MDAI) and inactivity (MDII) intervals independent of recording time, were measured. An activity interval was defined as a period during which the patient had more than 2 movements per minute, and an inactivity interval as a period during which the patient had less than 2 movements per minute. After 3 d, means of pooled data were calculated. Recording was made in hospitalized patients, so that physical activity was standardized.
Quantitative data were compared by ANOVA. Correlations were performed by Pearson test (P < 0.05).
There was no difference between the 3 groups according to venous ammonia (41 ± 17 vs 46 ± 33 vs 65 ± 50 μmol/L respectively) or VEP (N3 = 201 ± 31 vs 205 ± 38 vs 227 ± 70 milliseconds). Electroencephalography mean dominant frequency (MDF) correlated significantly with clinical stages of HE (r = 0.65, P = 0.003). The best correlation with HE stage was provided by actigraphy especially with MDAI (r = 0.7, P < 10-4) (Figure 1) and mesor (r = 0.65, P < 10-4). Moreover MDAI correlated significantly with MDF (r = 0.62, 0.004) and was significantly shorter in case of HE compared to patients without HE (stage 0: 5.33 ± 1.6 min versus stage 1-2: 3.28 ± 1.4 versus stage 3-4: 2.52 ± 1.1; P < 0.05). In stages 0 and 1-2 of HE, MDAI correlated significantly with Trail making test A (r = -0.61; P < 0.05). Using a threshold of MDAI of less than 4.9 min, sensitivity, specificity, positive predictive value, negative predictive value for HE diagnosis were 85%, 67%, 73% and 80%, respectively.
We suggest for the first time that actigraphy may be an objective method to identify HE. In our study, motor activity at the wrist recorded over three days, correlated well with clinical stages of HE. MDAI and mesor were the most relevant parameters. According to other works, neither serum ammonia[1] nor VEP[2] correlates with HE, contrary to EEG. However, EEG is a hospital procedure which has low specificity[3]. Using actigraphy, Cordoba et al[6] have previously shown a decrease of motor activity in cirrhotic patients without clinical evidence of HE. Seven out of 20 cirrhotic patients complained of unsatisfactory sleep, but they did not perform psychometric tests to screen subclinical encephalopathy. Actigraphy[4,5] is non invasive and simple to use. It can provide data under natural conditions over several days, which allows to take into account the oscillations in HE. One limit in our study is the decrease of motor activity due to bed-rest. However, we showed a significant relationship between actigraphy and encephalopathy. Actigraphy exhibits non specific changes in motor activity, but since we standardized physical activity, the observed changes might be due to HE. Our results suggest that actigraphy is especially fitted for early HE detection, since MDAI is significantly shorter in HE stage 1-2 patients compared to HE stage 0 patients. Moreover in cases of HE stages 0 and 1-2, MDAI, mesor, and acrophase correlate significantly with Trail making test A, a psychometric test routinely used to detect sub clinical encephalopathy. Further studies are needed to confirm actigraphy interest in sub clinical encephalopathy, a predictive factor of HE which is reversible by lactulose.
S- Editor Wang J L- Editor Wang XL E- Editor Bi L
| 1. | Basile AS, Jones EA. Ammonia and GABA-ergic neurotransmission: interrelated factors in the pathogenesis of hepatic encephalopathy. Hepatology. 1997;25:1303-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 72] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Sandford NL, Saul RE. Assessment of hepatic encephalopathy with visual evoked potentials compared with conventional methods. Hepatology. 1988;8:1094-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | van der Rijt CC, Schalm SW. Quantitative EEG analysis and evoked potentials to measure (latent) hepatic encephalopathy. J Hepatol. 1992;14:141-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Van Hilten JJ, Hoogland G, van der Velde EA, van Dijk JG, Kerkhof GA, Roos RA. Quantitative assessment of parkinsonian patients by continuous wrist activity monitoring. Clin Neuropharmacol. 1993;16:36-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Córdoba J, Cabrera J, Lataif L, Penev P, Zee P, Blei AT. High prevalence of sleep disturbance in cirrhosis. Hepatology. 1998;27:339-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 191] [Article Influence: 7.1] [Reference Citation Analysis (0)] |