Published online Apr 14, 2006. doi: 10.3748/wjg.v12.i14.2239
Revised: November 1, 2005
Accepted: November 10, 2005
Published online: April 14, 2006
AIM: To analyze our Wilson disease patient cohort (n = 106) for alterations in the gene coding for MURR1.
METHODS: Patients with an established diagnosis of Wilson disease but normal ceruloplasmin blood levels were chosen for our study (n = 14). Patients with two known disease-causing mutations in the ATP7B gene were not included. The three exons of the human MURR1 gene were sequenced after amplification of the genomic DNA by polymerase chain reaction.
RESULTS: Our study did not reveal any mutations leading to an amino acid change in the MURR1 sequence of Wilson disease patients. A polymorphism at 472 bp of the coding sequence could be confirmed.
CONCLUSION: The MURR1 gene plays no role in the pathogenesis of Wilson disease patients with normal serum ceruloplasmin levels.
- Citation: Weiss KH, Merle U, Schaefer M, Ferenci P, Fullekrug J, Stremmel W. Copper toxicosis gene MURR1 is not changed in Wilson disease patients with normal blood ceruloplasmin levels. World J Gastroenterol 2006; 12(14): 2239-2242
- URL: https://www.wjgnet.com/1007-9327/full/v12/i14/2239.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i14.2239
In humans, Wilson disease (WD) is an autosomal recessively inherited disorder of copper metabolism[1,2] characterized by the impaired biliary excretion of copper. Wilson disease leads to toxic copper accumulation predominantly in the liver and brain, causing liver cirrhosis and severe neurological defects. Common clinical findings in WD are low serum ceruloplasmin (CP) levels, elevated hepatic copper contents, elevated urine 24-h copper excretion and Kayser-Fleischer rings[3]. Homozygous or compound heterozygous mutations in the copper-transporting P-type ATPase ATP7B lead to Wilson disease[4,5].
The genetic background is highly variable, with more than 300 mutations reported so far[6]. But not all cases are unambiguous because no mutations in the ATP7B gene have been found in some WD patients. It is unclear why no ATP7B mutations are detectable in a subgroup of patients presenting with typical features of Wilson disease. It might be due to an incomplete analysis of the ATP7B gene or due to other yet unidentified defects of genes involved in copper metabolism.
The clinical presentation is highly variable even among patients with the same mutation. In Wilson disease a low serum ceruloplasmin level is a typical finding and can be observed in 80%-90% of the patients. Ceruloplasmin is a copper binding ferroxidase in blood[1]. Today’s understanding of the underlying molecular mechanisms[7] is that ATP7B is predominantly localized to the trans Golgi network and transports copper across the membrane to the lumen of the Golgi apparatus where apoceruloplasmin is loaded with copper. In case of a malfunction of ATP7B, apoceruloplasmin can not be loaded with copper and is degraded more rapidly, resulting in reduced blood levels of ceruloplasmin. Under elevated copper conditions, ATP7B translocates from the trans Golgi network to a vesicular compartment where it may facilitate biliary copper excretion[8,9].
Recently, the autosomal recessively inherited canine copper toxicosis has been described in Bedlington terriers. Like in Wilson disease these dogs develop copper accumulation in the liver due to impaired biliary copper excretion leading to chronic hepatitis and cirrhosis. Neurological abnormalities have not been reported. The genetic basis of this defect is a deletion of the exon2 of the Murr1 gene[10-12] mapped to 10q26 in Bedlington terriers. The human orthologous gene has been identified on chromosome 2p13-16 and is distinct from the ATP7B gene locus[11]. Furthermore, affected dogs present with normal ceruloplasmin serum levels, suggesting that the defect is beyond the trans-Golgi network. A direct interaction between MURR1 and ATP7B has been reported[13]. There is biochemical evidence that decreased MURR1 levels lead to intracellular copper accumulation[14]. Based on these observations a role of MURR1 in the biliary copper excretion downstream of ATP7B has been suggested[15].
It would be interesting to identify a human disorder caused by defects in the human MURR1 gene. Recently, a novel protein family (COMMD proteins) of structural and functional homologues of MURR1 (COMMD1) has been identified[16]. Recently, we reported an association between an MURR1 polymorphism and onset of neurological and hepatic symptoms in WD patients homozygous for the most common ATP7B mutation H1069Q. Onset of disease was significantly earlier in patients with a heterozygous state at codon Asn 164 (GAT/GAC) than in patients with wild type (GAT/GAT)[17]. In the former study patients with low ceruloplasmin serum levels were included.
To identify possible disease related mutations in the MURR1 gene in the current study we focused on patients with Wilson Disease but normal ceruloplasmin serum levels and at least one unknown mutation of ATP7B.
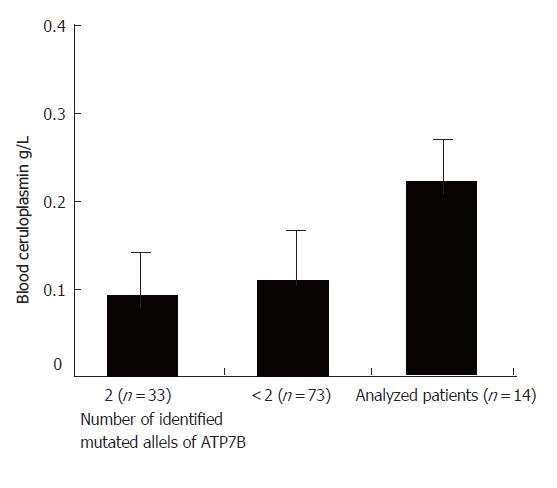
Data of patients with an established diagnosis of Wilson disease were collected (n = 106). The diagnosis of Wilson disease was based on the criteria of the 8th International Conference on Wilson Disease and Menkes disease[19] (Leipzig, Germany April 16-18, 2001). For this study only patients with at least one undetermined mutation of the ATP7B gene were selected (n = 73), patients with two known disease defining mutations were excluded (n = 33). The ATP7B gene was sequenced in most patients in cooperation with Professor Ferenci, Department of Gastroenterology and Hepatology, Vienna, including the H1069Q mutation state in all patients. For our investigation out of the subset of WD patients without or with only one disease defining mutation, patients with normal or only slightly reduced ceruloplasmin levels were selected (n = 14) at the beginning of the study.
Ceruloplasmin cutoff was defined at a CP level of 0.15 g/L (normal in healthy persons: 0.2-0.6 g/L). Patients were excluded if the finding of a CP level above 0.15 g/L could be explained otherwise (e.g. contraceptive medication, pregnancy, acute phase reaction). A total of 14 patients with a CP level > 0.15 g/L and without any or with only one ATP7B mutation could be identified (Figure 1).
Mutation analysis of MURR1 was performed as described in detail previously[17]. In short, total DNA from the 14 selected patients was isolated and the three exons of the MURR1 gene were amplified by polymerase chain reaction. The used primer combinations (exon 1 sense primer 5‘-GGT GGT TTT GCA CAG GCT ATT TAG-3‘, exon 1 anti-sense primer 5‘-GGC TTG TGA GGA CAG GGG AAG G-3‘; exon 2 sense primer 5‘-CAG TGA TTT AAG AGT CAC TC-3‘, exon 2 anti-sense primer 5‘-GCT GAA TAG ACA AGC TAA CAT GTA-3‘; exon 3 sense primer 5‘-GGG TAT TTT GAG TTT GGT CAT GC-3‘, exon 3 anti-sense primer 5’-TGA GAA CCT CTG CAC TGG AAC-3‘) resulted in PCR products covering the exons and parts of the 3` untranslated region and parts of the 5` region upstream of the start codon[17]. Additional putative exons or putative regulatory regions of the MURR1 gene were not analyzed.
PCR products were purified as described previously[17]. Sequencing reactions were carried out by SEQLAB (Sequence Laboratories, Goettingen, Germany) or by MPI sequencing (Max Planck Institute, Dresden, Germany). For later analysis NM-152516 and AB17881 (NCBI sequence Viewer, http://www.ncbi.nlm.nih.gov/) were used as reference sequence. Nucleotide changes were numbered corresponding to their position in the MURR1 mRNA beginning with the adenine of the ATG start codon.
The human orthologue of the Murr1 gene encoded a protein of 190 amino acids. The gene spanned nearly 235 kb. Both introns were about 100 kb each in size (Figure 2). Therefore only the three exonic sequences were analyzed. In this study no mutations changing the amino acid sequence were found in the analyzed patients. A polymorphism at 472 bp of the coding sequence was detected (Table 1). Ten Patients (71%) were homozygous for wild-type GAT, 3 patients (21%) were heterozygous GAT/GAC and 1 patient was homozygous for GAC. The frequency of these variations was in line with previous reports[17]. We already reported a putative association between the GAT/GAC heterozygous state at codon Asn-164 with an earlier onset of disease in H1096Q ATP7B homozygous patients [17]. However, in the present study no significant genotype/phenotype correlation could be found, which might be due to the small number of patients.
| Mutation in the ATP7B gene | n | MURR1 gene base changes | CP level | ||
| GAT/GAT | GAT/GAC | GAC/GAC | |||
| 2299InsC/ m n d. | 1 | 1 | - | - | 0.15 |
| 3400DelC/ m n d. | 1 | 1 | - | - | 0.18 |
| G1030C/ m n d. | 1 | 1 | - | - | 0.28 |
| H1069Q/ m n d. | 1 | - | - | 1 | 0.31 |
| m.n.d./ m n d. | 7 | 7 | - | - | 0.23 (± 0.09) |
| m.n.d./ m n d. | 3 | - | 3 | - | 0.26 (± 0.08) |
Some patients with Wilson disease show no disease causing mutation in the ATP7B gene. Therefore other pathogenetic factors might be involved. Due to the in part comparable phenotype of canine copper toxicosis and the reported interaction with ATP7B, the MURR1 protein is an interesting candidate.
In this study we focused on Wilson disease patients showing a comparable phenotype to the copper toxicosis in dogs in regard to the normal ceruloplasmin serum level. However, in our group of patients with non-homozygous ATP7B mutation, no mutation in the coding sequence of the MURR1 gene was found. Mutations in other parts of the Murr1 gene were not analyzed but could affect the gene expression and thus affect the phenotype. The finding that copper toxicosis gene MURR1 is not changed in Wilson disease patients with normal blood ceruloplasmin levels needs to be evaluated in a larger clinical study.
Although our data do not necessarily rule out the possibility that MURR1 is involved in biliary copper excretion under physiological conditions, there is no direct evidence that it is indeed a disease-causing factor in human Wilson disease. This is in agreement with the findings of other investigators[17,18]. In fact, a homozygous mutation of ATP7B resulting in the absence of this protein in the trans-Golgi network may be the only cause of Wilson disease. In our study population with detectable heterozygous or absent mutations of ATP7B, the other mutations may have not been identified yet. A low normal ceruloplasmin concentration was also observed in patients with homozygous mutations, suggesting that it may represent an undefined compensatory process by which apoceruloplasmin is loaded with copper by an ATP7B independent route, even outside the trans-Golgi network. This route seems to be less efficient but could result in low normal serum coeruloplasmin levels.
Although the possibility that MURR1 is involved in biliary copper excretion in humans has not been ruled out, MURR1 does not seem to play a role in the pathogenesis of Wilson disease.
The authors thank the patients for their help and willingness to participate in this study and Dr. Bettina Stuehler for her advice and help with the sequencing reactions.
S- Editor Pan BR L- Editor Wang XL E- Editor Cao L
| 1. | Harris ED. Cellular copper transport and metabolism. Annu Rev Nutr. 2000;20:291-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 193] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 2. | Gitlin JD. Wilson disease. Gastroenterology. 2003;125:1868-1877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 261] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 3. | Riordan SM, Williams R. The Wilson's disease gene and phenotypic diversity. J Hepatol. 2001;34:165-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 90] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Bull PC, Thomas GR, Rommens JM, Forbes JR, Cox DW. The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nat Genet. 1993;5:327-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1446] [Cited by in RCA: 1311] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 5. | Tanzi RE, Petrukhin K, Chernov I, Pellequer JL, Wasco W, Ross B, Romano DM, Parano E, Pavone L, Brzustowicz LM. The Wilson disease gene is a copper transporting ATPase with homology to the Menkes disease gene. Nat Genet. 1993;5:344-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 991] [Cited by in RCA: 909] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 6. | Available from URL: http: //www; medgen.med.ualberta.ca/database.html. . |
| 7. | Lutsenko S, Petris MJ. Function and regulation of the mammalian copper-transporting ATPases: insights from biochemical and cell biological approaches. J Membr Biol. 2003;191:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 154] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 8. | Schaefer M, Roelofsen H, Wolters H, Hofmann WJ, Müller M, Kuipers F, Stremmel W, Vonk RJ. Localization of the Wilson's disease protein in human liver. Gastroenterology. 1999;117:1380-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 60] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Roelofsen H, Wolters H, Van Luyn MJ, Miura N, Kuipers F, Vonk RJ. Copper-induced apical trafficking of ATP7B in polarized hepatoma cells provides a mechanism for biliary copper excretion. Gastroenterology. 2000;119:782-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 203] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 10. | van De Sluis B, Rothuizen J, Pearson PL, van Oost BA, Wijmenga C. Identification of a new copper metabolism gene by positional cloning in a purebred dog population. Hum Mol Genet. 2002;11:165-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 277] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 11. | van de Sluis BJ, Breen M, Nanji M, van Wolferen M, de Jong P, Binns MM, Pearson PL, Kuipers J, Rothuizen J, Cox DW. Genetic mapping of the copper toxicosis locus in Bedlington terriers to dog chromosome 10, in a region syntenic to human chromosome region 2p13-p16. Hum Mol Genet. 1999;8:501-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Klomp AE, van de Sluis B, Klomp LW, Wijmenga C. The ubiquitously expressed MURR1 protein is absent in canine copper toxicosis. J Hepatol. 2003;39:703-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 109] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Tao TY, Liu F, Klomp L, Wijmenga C, Gitlin JD. The copper toxicosis gene product Murr1 directly interacts with the Wilson disease protein. J Biol Chem. 2003;278:41593-41596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 124] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 14. | Burstein E, Ganesh L, Dick RD, van De Sluis B, Wilkinson JC, Klomp LW, Wijmenga C, Brewer GJ, Nabel GJ, Duckett CS. A novel role for XIAP in copper homeostasis through regulation of MURR1. EMBO J. 2004;23:244-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 177] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 15. | Wijmenga C, Klomp LW. Molecular regulation of copper excretion in the liver. Proc Nutr Soc. 2004;63:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 57] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Burstein E, Hoberg JE, Wilkinson AS, Rumble JM, Csomos RA, Komarck CM, Maine GN, Wilkinson JC, Mayo MW, Duckett CS. COMMD proteins, a novel family of structural and functional homologs of MURR1. J Biol Chem. 2005;280:22222-22232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 216] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 17. | Stuehler B, Reichert J, Stremmel W, Schaefer M. Analysis of the human homologue of the canine copper toxicosis gene MURR1 in Wilson disease patients. J Mol Med (Berl). 2004;82:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Müller T, van de Sluis B, Zhernakova A, van Binsbergen E, Janecke AR, Bavdekar A, Pandit A, Weirich-Schwaiger H, Witt H, Ellemunter H. The canine copper toxicosis gene MURR1 does not cause non-Wilsonian hepatic copper toxicosis. J Hepatol. 2003;38:164-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Ferenci P, Caca K, Loudianos G, Mieli-Vergani G, Tanner S, Sternlieb I, Schilsky M, Cox D, Berr F. Diagnosis and phenotypic classification of Wilson disease. Liver Int. 2003;23:139-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 717] [Cited by in RCA: 603] [Article Influence: 27.4] [Reference Citation Analysis (0)] |