Published online Apr 14, 2006. doi: 10.3748/wjg.v12.i14.2209
Revised: October 15, 2005
Accepted: November 18, 2005
Published online: April 14, 2006
AIM: To clarify the innervation of the antro-pyloric region in humans from a clinico-anatomical perspective.
METHODS: The stomach, duodenum and surrounding structures were dissected in 10 cadavers, and immersed in a 10mg/L solution of alizarin red S in ethanol to stain the peripheral nerves. The distribution details were studied to confirm innervations in the above areas using a binocular microscope. Similarly, innervations in 10 Suncus murinus were examined using the method of whole-mount immunohistochemistry.
RESULTS: The innervation of the pyloric region in humans involved three routes: One arose from the anterior hepatic plexus via the route of the suprapyloric/supraduodenal branch of the right gastric artery; the second arose from the anterior and posterior gastric divisions, and the third originated from the posterior-lower region of the pyloric region, which passed via the infrapyloric artery or retroduodenal branches and was related to the gastroduodenal artery and right gastroepiploic artery. For Suncus murinus, results similar to those in humans were observed.
CONCLUSION: There are three routes of innervation of the pyloric region in humans, wherein the route of the right gastric artery is most important for preserving pyloric region innervation. Function will be preserved by more than 80% by preserving the artery in pylorus-preserving pancreaticoduodenectomy (PPPD). However, the route of the infrapyloric artery should not be disregarded. This route is related to several arteries (the right gastroepiploic and gastroduodenal arteries), and the preserving of these arteries is advantageous for preserving pyloric innervation in PPPD. Concurrently, the nerves of Latarjat also play an important role in maintaining innervation of the antro-pyloric region in PPPD. This is why pyloric function is not damaged in some patients when the right gastric artery is dissected or damaged in PPPD.
- Citation: Yi SQ, Ru F, Ohta T, Terayama H, Naito M, Hayashi S, Buhe S, Yi N, Miyaki T, Tanaka S, Itoh M. Surgical anatomy of the innervation of pylorus in human and Suncus murinus, in relation to surgical technique for pylorus-preserving pancreaticoduodenectomy. World J Gastroenterol 2006; 12(14): 2209-2216
- URL: https://www.wjgnet.com/1007-9327/full/v12/i14/2209.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i14.2209
With the concept of less invasive surgery, pylorus-preserving pancreaticoduodenectomy (PPPD) has taken the place of conventional Whipple pancreaticoduodenectomy as a standard operative procedure for the treatment of various benign and malignant diseases in the periampullary region, even pancreatic head carcinoma, since 1978[1-5]. Early delayed gastric emptying has been described as a common and frustrating complication after this procedure, which occurs in 20% to 46% of patients[6-16]. Although delayed gastric emptying is self-limiting, not life-threatening, and can be treated conservatively[7,9,17], it results in discomfort and a significant prolonging of the hospital stay, and so contributes to increased hospital costs[7,8,18,19]. However, the pathogenesis of delayed gastric emptying after PPPD remains controversial.
The procedure may impair gastric peristalsis. The duodenal pacemaker, which is located 0.5 to 1 cm distally from the pylorus, should therefore be preserved to avoid disturbances in normal gastric peristalsis[20]. Gastric arrhythmias may be another cause of delayed gastric emptying in the early postoperative period, probably exacerbated by intra-abdominal complications[21]. Problems caused by the surgical procedure itself include injury to the nerves of Latarjat or the placement of suture material through the pyloric muscle. In addition, ischemia of the duodenal stump and antropyloric muscle mechanisms could influence gastric emptying, although mucosal edema at the site of anastomosis and peri-anastomotic fluid collection seem to be more common problems[22]. In regard to maintaining the pylorus as a functional unit, it is of paramount importance to maintain normal innervation of the antro-pylorus, an adequate blood supply and a pyloric muscle unimpeded by sutures[1,23].
Moreover, there has been no detailed description concerning clinico-anatomical and morphologic studies of the innervation of the antro-pyloric region in other literature, although there are some records of human pyloric innervation in literatures[24-27]. Therefore, we attempted to clarify the innervation of the antro-pyloric region in humans from a clinico-anatomical point of view, and to evaluate the innervation-preserving procedure of PPPD and its modified procedures, by using the peculiar method of labeling and dissecting the autonomic nerves of the viscera, not the current dissection of gross anatomy as in our previous studies[28-30]. Furthermore, the experimental animal, Suncus murinus, has been used for a comparative study to demonstrate general morphologic characteristics more similar to humans than other current laboratory animals, e.g., mouse, rat and rabbit[28-31].
The study was performed on 10 cadavers (5 men and 5 women) with a mean age of 79.8 (range, 50 to 94) years. All cadavers were selected from bodies used for research and practice of anatomy at Kanazawa University School of Medicine during the years 1999-2000, and were free from diseases of the liver, stomach, duodenum, pancreas, and their surrounding areas (Table 1).
| Case | Sex | Age | Death | Number1 | Route I | Route II | Route III |
| A | F | 94 | Pneumonia | 1 | O | O | |
| B | F | 81 | Cerebral hemorrhage | 2 | O | O | O |
| C | F | 83 | Myocardial infarction | 1 | O | O | |
| D | M | 87 | Myocardial infarction | 4 | O | O | O |
| E | M | 86 | Pneumonia | 4 | O | O | |
| F | M | 73 | Cerebral hemorrhage | 3 | O | O | O |
| G | M | 87 | Cerebral hemorrhage | 4 | O | O | |
| H | M | 75 | Myocardial infarction | 4 | O | O | |
| I | F | 82 | Subarachnoid hemorrhage | 5 | O | O | O |
| J | F | 50 | Pneumonia | 3 | O | O | O |
Adult laboratory house musk shrews, S. murinus, were obtained and maintained from a closed breeding colony bred in our laboratories in the Department of Anatomy and Neuroembryology, Kanazawa University, Japan. The animals were housed and handled in accordance with the Guide for the Care and Use of Laboratory Animals and the Guide for the Care and Use of Experimental Animals of the Canadian Council on Animal Care. Briefly, all shrews were kept individually after weaning (20 d after birth) in plastic cages equipped with a wooden nestbox containing paper strips, and were kept in a conventionally conditioned animal room: 23 °C to 27 °C, no humidity control, and 14 L: 10 D. Commercial trout pellets containing 45.0% protein, 3.5% fat, 3.0% fiber, 13.0% ash and 26.2% complex carbohydrate (Nippon Haigou Shiryou, Tokyo, Japan) and water were supplied ad libitum. The mother colony, JIc: CR, is maintained in the Central Institute for Experimental Animals, Kawasaki, Japan[28]. Adult animals (6 females and 4 males, weighing 45-80 g) were first anesthetized with ether and received an intraperitoneal injection of a solution of urethrane (sodium ethyl carbamate, 900 mg/kg). After each S. murinus was completely narcotized, the abdominal cavity was opened, and a catheter was inserted retrogradely into the abdominal aorta at the level immediately above the bifurcation of this artery into the common iliac arteries. Perfusion was commenced with normal saline containing heparin (10 KU/L), and thereafter with phosphate-buffered saline (PBS) containing 40 g/L paraformaldehyde. After perfusion, the animals were injected with neoprene latex to label the blood vessels in the pylorus region. Thereafter, the abdominal organs including the stomach, duodenum, common bile duct, and pancreas were extracted en bloc with the related nerves and vessels, postfixed with 40 g/L paraformaldehyde in PBS (pH 7.4) at 4 °C overnight to prepare for whole mount immunostaining.
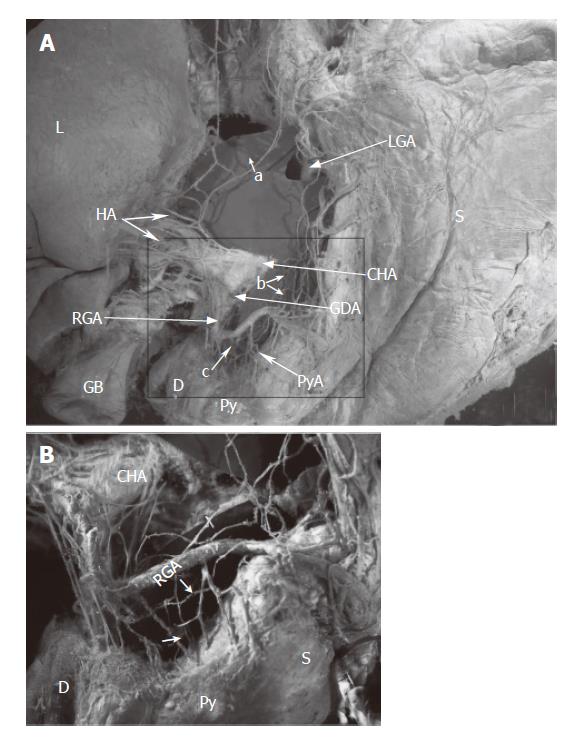
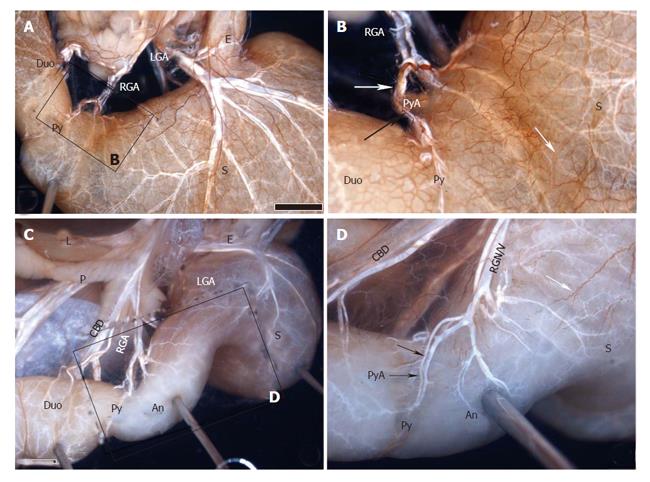
The anatomical procedures of the cadavers were performed, according to our previous description[29,30], as follows. From the adult autopsy, the viscera of the upper abdomen (including the liver, pancreas, lower esophagus, stomach, and duodenum) were resected en masse with the abdominal aorta (region including the celiac artery and superior mesenteric artery), portal system, and nerves (including the vagus nerve, celiac ganglion, and plexus). The resected specimens were immersed in a 10 mg/L solution of alizarin red S (Wako, Osaka, Japan) in ethanol to melt the fat tissue and stain the peripheral nerves. The solution was changed 3 times every 2 to 3 d, in principle, but this process may be prolonged if necessary depending on the degree of progression of elimination of fat and staining. The area of each sample surrounded by the horizontal plane that passed through the portal region and the lower margin of the horizontal part of the duodenum and the sagittal plane that passed through the descending part of the duodenum and hilum of the spleen was dissected to the depth of the celiac plexus with the aid of a stereoscopic microscope (magnification, × 40), keeping the sample immersed completely in 100% ethanol. In dissection, the lymphatic vessels and lymph nodes were removed, and particular attention was paid to preserve not only the nerves but also the arteries and veins around the stomach including the hepatogastro mesenteriolum, hepatoduodenal ligament and lower esophagus. Figures 1 and 2 show two such dissections.
The whole mount immunostaining procedures for the S. murinus were performed as previously described[28,30]. Briefly, after rinsing in PBS, the fixed specimens were treated with 10 g/L periodic acid for 20 min to prevent any intrinsic peroxidase reaction. They were then incubated in freshly prepared 5 g/L papain (Sigma) in 0.025 mol/L Tris-HCl buffer (pH7.6) for 1 h and 25 g/L, 50 g/L, and 100 g/L sucrose in PBS for 30 min, respectively, followed by freezing and thawing thrice. The specimens were incubated with primary antibody (NFP-Ab) in PBS containing 2 g/L bovine serum albumin (BSA), 3 g/L Triton X-100, and 1 g/L sodium azide for 3 d at 4 °C. After a thorough wash in PBS, the specimens were then incubated with secondary antibody labeled with peroxidase-conjugated affinity-purified sheep anti-mouse IgG (HRP) in PBS containing 2 g/L BSA and 3 g/L Triton X-100 for 3 d at 4 °C. After a thorough wash in PBS, coloration was performed in 0.05 mol/L Tris-HCl buffer containing 20 mg/L 3,3'-diaminobenzidine (DAB) and 0.1 ml/L H2O2 for 1 to 3 d at 4 °C. The stained preparations were then stored in glycerin to obtain transparency. The primary antibody was anti-neurofilament protein (NFP) antibody, a monoclonal mouse anti-all neurofilament consisting of three subunit proteins: NF-H (200 ku), NF-M (160 ku), and NF-L (70 ku) (M0762, lot 089, clone: 2F11, Dako).
The innervation of the pyloric region in human involved three routes: via the right gastric artery, the nerves of Latarjet, and the infrapyloric artery.
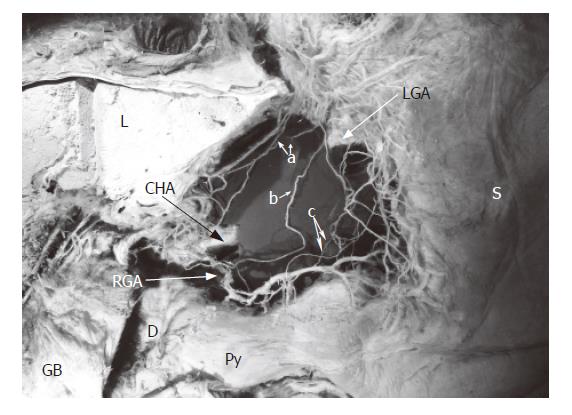
The hepatic division of the vagus, arising from the anterior vagal trunk, ran through the hepatogastric ligament near the edge of the liver (caudal liver), and joined the anterior hepatic plexus in the hepatoduodenal ligament. The plexus, containing the parasympathetic and sympathetic fibers (the latter originate from the celiac plexus), wound around the proper hepatic artery, then the suprapyloric or supraduodenal branch which comes from the right gastric artery, sent some branches to the pyloric region in the superior part of the pyloric regions (Figures 1, 2). This route was displayed in all ten specimens.
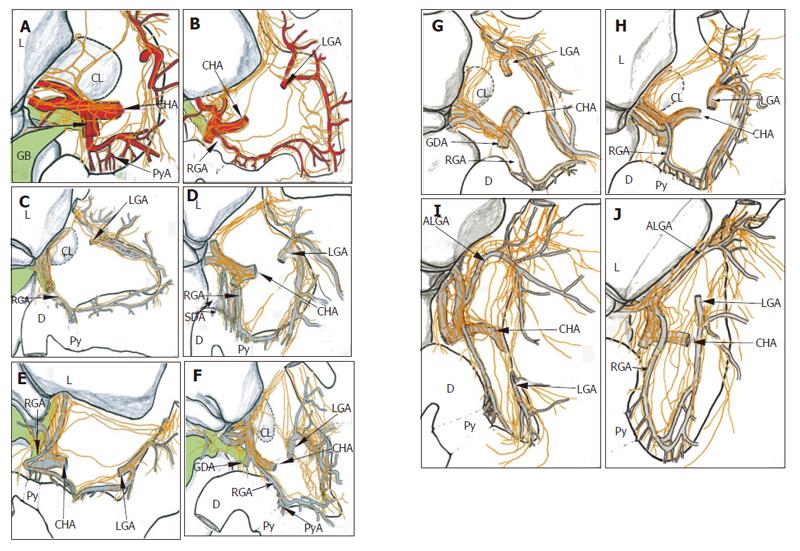
There was variation in the number of hepatic divisions. The total number of hepatic divisions was 46 (average, 4.6) in the 10 specimens in this study. The position where the hepatic division joined the anterior hepatic plexus occurred at different levels between the hepatic hilum and the root of the right gastric artery(Table 1, Figure 3). There were 2 cases of accessory left gastric artery in the 10 specimens. The hepatic division ran along the arteries to the hepatic hilum in these 2 cases(Figures 3I and 3J).
The branches, arising from the origin of the hepatic division, sent single or double descending branches of the hepatic division, ran in the lesser omentum, did not reach the proper hepatic artery or join the anterior hepatic plexus, joined directly to the right gastric artery, and then entered the pyloric region. Five cases of this route were observed in the 10 cadavers (Table 1, Figures 3B, 3D, 3F, 3I, and 3J).
The anterior and posterior gastric divisions, namely the nerves of Latarjet, extended to and ran along the gastric lesser, while giving off some offshoots to the lesser curvature, sending some peripheral nerve fibers to the antro-pyloric region. The nerves of Latarjet passed through the lesser omentum, which lies 0.5 to 1.0 cm from the lesser curvature, or extended and anastomosed with the offshoots along the right gastric artery, or lay beneath the serosa of the gastric wall, entered the antro-pyloric region. The route was observed in all cases (Table 1, Figures 1, 2, and 3).
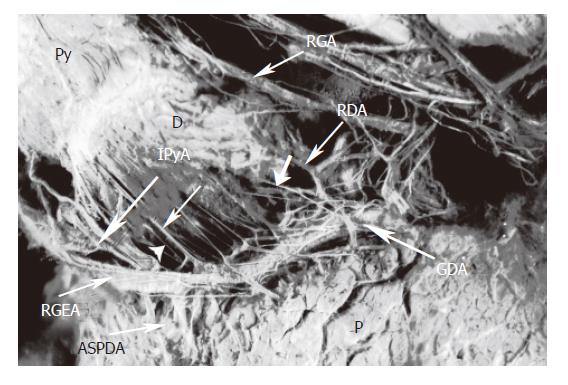
There is always one or several infrapyloric arteries or/and retroduodenal branches, intended for the posterior-lower region of the pylorus. All of these arteries arise either from the gastroduodenal artery, or from one or the other of these two terminal branches, the right gastroepiploic artery, or the cranial and ventral pancreaticoduodenal artery.
This route with the arteries supplying the posterior-lower region of the pylorus, passed the nerve branches to the pyloric region. The nerve branches, arising from the anterior hepatic plexus, ran along the gastroduodenal artery, or the right gastroepiploic artery, to the infrapyloric artery or/and retroduodenal branches, reached the posterior-lower region of the pylorus. The route was observed in all cases (Figure 4).
Firstly, it is necessary to describe the morphological characters of the pyloric region in S. murinus. The pylorus closed the duodenal papilla, supplied by the branches of the gastroduodenal artery. The latter sent the branches to the duodenal papilla and the pyloric region. The arterial branch that supplied the pyloric region corresponded to the right gastric artery in humans. However, there existed no ramus anastomoticus between this artery and the left gastric arteries in S. murinus (Figure 5). There was the lesser omentum in this animal, and the right gastric artery sent anterior and posterior branches to the anterior and posterior of the pyloric region. Furthermore, there was no infrapyloric artery arising from the gastroduodenal artery in contrast to humans. Even though there are these differences, the innervation of the pyloric region in S. murinus was very similar to that in humans.
In S murinus, the nerves originating from hepatic plexus, ran along the gastroduodenal artery, the right gastric artery, and reached the pyloric region. This route was simple, and did not form the ramus anastomoticus with the anterior or posterior gastric branches, which originate from the vagal trunk or the left gastric artery. The innervation was observed in all cases (Figure 5).
In S murinus, the anterior and posterior gastric branches, corresponding to the Latarjet nerves in humans, while giving off some divisions to the lesser curvature and the gastric wall, sent some branches to the pyloric regions in all cases (Figure 5). The posterior-lower region of the pylorus in S murinus was innervated by nerve branches originating from the right gastric artery, in constrast to humans. The anterior and posterior gastric branches ran along the lesser omentum and close to the lesser curvature, or lay beneath the serosa of the gastric wall. Similarly, the route passing through the lesser omentum was also observed in some specimens, and these nerves originated from the anterior or posterior vagal trunk directly. The innervation was observed in all cases (Figure 5).
The present paper is concerned with a detailed observation of the innervation of the antro-pyloric region, with the objective of providing an anatomical basis for surgical operations in this region, especially concerning ‘organ and function-preserving surgical procedures’ like PPPD.
There are three routes of pyloric innervation in humans. One is the superior region of the pylorus, which is related to the hepatoduodenal ligament, whereby the nerve branches arose from the anterior hepatic plexus containing the branches coming from the hepatic division of vagus. The nerves ran along the right gastric artery, via the suprapyloric or supraduodenal branch, intended for the antro-pyloric region. The second route is the posterior-lower region of the pylorus, which is related to the gastro-pancreatic ligament. The nerves ran along the gastroduodenal or right gastroepiploic artery, to the infrapyloric artery, and reached the antro-pyloric region. The third route is the lower antrum region, which is related to the left gastric artery and the nerves of Latarjet. This route involves the branches of Latarjet nerves passing through the lesser curvature, and entering the antro-pyloric region. The animal model, S murinus, used in this study, allowed the complete anatomic observation of cadavers, exhibited the important arterial supply routes of the pylorus and anterior and posterior gastric divisions for the innervation of the pylorus.
The route of the superior region is the most important for pyloric innervation, in which the right gastric artery shows a core mission (main rule). The nerves of this route contain both sympathetic and parasympathetic fibers, originating from either the anterior hepatic plexus which arise from the celiac plexus, or the hepatic division of the anterior vagal trunk which joins the proper hepatic artery to the right gastric artery. The route was observed in all 10 cadavers. There were 4 cases of hepatic division distal to the hepatic edge, the division passing through the hepatogastric ligament, terminating in the right gastric artery in 10 cadavers. But only one case showed the pattern illustrated by McCrea[24], whereby a single descending branch of the hepatic division directly reached the pylorus. We agree with Skandalakis et al[27] that this pattern is typical but not universal. Namely, pyloric branches of the hepatic division do not, usually, directly reach the pyloric region, but pass via the arterial supply of the region, as the right hepatic artery and its branches.
The right gastric artery arises from the hepatic artery and proceeds to the first portion of the duodenum (the supraduodenal artery), the pylorus and the antrum (the suprapyloric branch) along the lesser curvature. These routes via these arteries are important for pyloric innervation via the superior region of the pylorus. The preservation of the right gastric artery and its branches is potentially of great importance in providing earlier gastric emptying after operation. The drawback of pyloric preservation is a prolonged period of gastric suction. This time can be shortened by attention to the preservation of the supraduodenal artery and vagal innervation of the antrum, which is essential for gastric emptying[7]. In any event, as there was a reliable route of the right gastric artery, it is not necessary to preserve the hepatogastric ligament in order to preserve the route in clinic practice. In principle, it is only important to preserve the right gastric artery in order to preserve the innervation and function of the pylorus in PPPD.
For the route of the nerves of Latarjet, innervation originating from the anterior and posterior gastric branches of the nerves of Latarjet passed through the lesser omentum close to the lesser curvature, or extended to and anastomosed with the offshoots along the right gastric artery, or lay beneath the serosa of the gastric wall, and entered the antro-pyloric region. Skandalakis et al [26] reported that the nerves of Latarjet could be traced distally to about the level of the incisura in most specimens, but in many cases it reached the pylorus, and in six cases it was visible as far as the first part of the duodenum. It is possible that the nerves of Latarjet innervate the antro-pyloric region according to our observations in humans and S. murinus. Great care must be taken to preserve the blood supply from the left gastric artery to the lesser curvature of the stomach and pylorus in PPPD.
For the innervation of the posterior-lower region of the pylorus, it was disregard ordinarily. The route is via the arterial supply of the posterior-lower region of the pylorus. The branches are sent off by the gastroduodenal or the right gastroepiploic artery, termed by the infrapylorus branch or artery, or the retroduodenal branches. These branches come from the gastroduodenal artery in 30% of cases, from the right gastroepiploic artery in 44% of cases or from the ventral pancreaticoduodenal arch in 20% of cases[32]. The gastroduodenal artery should probably be severed distally to its first branch and should be preserved in cases of chronic pancreatitis where dissection could proceed by taking the pancreaticoduodenal artery at its origin from the gastroduodenal artery. Equal attention must also be given during the procedure to preserving innervations to the antrum and pylorus[9]. The gastroepiploic artery, with its large ascending pyloric branch, should probably be taken close to its origin from the gastroduodenal artery[9]. The origin of the innervation in this route is similar to that of the superior region, coming from the anterior hepatic plexus. Hence, the innervation also contained both the sympathetic and parasympathetic fibers. If the right gastric artery is damaged, the route is able to compensate by its function in innervation. Grace et al [33] emphasized that an intact neurovascular supply to the pylorus and the first part of the duodenum is essential for the success of the pylorus preserving operation. By not ligating the gastroduodenal artery and right gastric artery at their origins and not freeing the tissues along the lesser curvature of the antrum and the gastrohepatic ligament-all these tissues remaining intact-the surgeon cannot rotate the proximal duodenum and antrum anteriorly and to the patient's left in PPPD. Thereby, it is advantageous to preserve all blood supply and innervation to antrum, pylorus, and proximal duodenum[16]. In fact, innervation of the antro-pyloric region takes several routes. When dissecting the lymph nodes in the hepatoduodenal ligament, the nerve branches originating from the anterior hepatic plexus to the right gastric artery could be damaged, however, as there are other routes of the posterior-lower region of the pylorus and the nerves of Latarjet, their influence on pyloric function may often be unclear.
In conclusion, we demonstrated the detailed description of the innervation of the antro-pyloric region from clinico-anatomical and morphologic perspectives in this study. The useful methods of whole mount immunostaining with a peripheral neuron marker for S murinus, and the alizarin red S staining technique for humans are effective for peripheral nerve labeling, as shown in our studies. There are three routes of pyloric innervation in humans, wherein the route of the right gastric artery is the most important for preserving pyloric region innervation. The function is preserved by more than 80% by preserving the artery in PPPD. However, the route of the infrapyloric artery should not be disregarded. This route is related to several arteries (the right gastroepiploic and gastroduodenal arteries), and the preservation of these arteries is advantageous for preserving pyloric innervation in PPPD. Concurrently, the nerves of Latarjet also perform an important role in maintaining innervation of the antro-pyloric region in PPPD. This is why the pyloric function is not damaged in some patients if the right gastric artery is dissected or damaged in PPPD.
We thank Mr. Nakamura, Mr. Shiraishi and Mrs. Koizumi (Department of Anatomy and Neuroembryology, Kanazawa University) for their technical assistance, and Mrs Ogawa (Department of Anatomy, Tokyo Medical University) for her secretarial assistance during this study.
S- Editor Pan BR L- Editor Zhang JZ E- Editor Cao L
| 1. | Traverso LW, Longmire WP. Preservation of the pylorus in pancreaticoduodenectomy. Surg Gynecol Obstet. 1978;146:959-962. [PubMed] |
| 2. | Ueno T, Tanaka A, Hamanaka Y, Tsurumi M, Suzuki T. A proposal mechanism of early delayed gastric emptying after pylorus preserving pancreatoduodenectomy. Hepatogastroenterology. 1995;42:269-274. [PubMed] |
| 3. | Nagai H, Ohki J, Kondo Y, Yasuda T, Kasahara K, Kanazawa K. Pancreatoduodenectomy with preservation of the pylorus and gastroduodenal artery. Ann Surg. 1996;223:194-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Yamaguchi K, Kishinaka M, Nagai E, Nakano K, Ohtsuka T, Chijiwa K, Tanaka M. Pancreatoduodenectomy for pancreatic head carcinoma with or without pylorus preservation. Hepatogastroenterology. 2001;48:1479-1485. [PubMed] |
| 5. | Sugiyama M, Abe N, Ueki H, Masaki T, Mori T, Atomi Y. A new reconstruction method for preventing delayed gastric emptying after pylorus-preserving pancreatoduodenectomy. Am J Surg. 2004;187:743-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Braasch JW, Gongliang J, Rossi RL. Pancreatoduodenectomy with preservation of the pylorus. World J Surg. 1984;8:900-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Braasch JW, Deziel DJ, Rossi RL, Watkins E, Winter PF. Pyloric and gastric preserving pancreatic resection. Experience with 87 patients. Ann Surg. 1986;204:411-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 191] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Warshaw AL, Torchiana DL. Delayed gastric emptying after pylorus-preserving pancreaticoduodenectomy. Surg Gynecol Obstet. 1985;160:1-4. [PubMed] |
| 9. | Itani KM, Coleman RE, Meyers WC, Akwari OE. Pylorus-preserving pancreatoduodenectomy. A clinical and physiologic appraisal. Ann Surg. 1986;204:655-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 130] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Aonuma K. [Experimental studies on the gastroduodenojejunal motility and gastric emptying after duodenectomy with preservation of the total stomach and pylorus]. J Smooth Muscle Res. 1994;30:147-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Yeo CJ, Cameron JL, Sohn TA, Lillemoe KD, Pitt HA, Talamini MA, Hruban RH, Ord SE, Sauter PK, Coleman J. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg. 1997;226:248-57; discussion 257-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1357] [Cited by in RCA: 1387] [Article Influence: 49.5] [Reference Citation Analysis (34)] |
| 12. | Müller MW, Friess H, Beger HG, Kleeff J, Lauterburg B, Glasbrenner B, Riepl RL, Büchler MW. Gastric emptying following pylorus-preserving Whipple and duodenum-preserving pancreatic head resection in patients with chronic pancreatitis. Am J Surg. 1997;173:257-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 68] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Yamaguchi K, Tanaka M, Chijiiwa K, Nagakawa T, Imamura M, Takada T. Early and late complications of pylorus-preserving pancreatoduodenectomy in Japan 1998. J Hepatobiliary Pancreat Surg. 1999;6:303-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 101] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Murakami H, Yasue M. A vertical stomach reconstruction after pylorus-preserving pancreaticoduodenectomy. Am J Surg. 2001;181:149-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Murakami H, Suzuki H, Nakamura T. Pancreatic fibrosis correlates with delayed gastric emptying after pylorus-preserving pancreaticoduodenectomy with pancreaticogastrostomy. Ann Surg. 2002;235:240-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Gauvin JM, Sarmiento JM, Sarr MG. Pylorus-preserving pancreaticoduodenectomy with complete preservation of the pyloroduodenal blood supply and innervation. Arch Surg. 2003;138:1261-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Patel AG, Toyama MT, Kusske AM, Alexander P, Ashley SW, Reber HA. Pylorus-preserving Whipple resection for pancreatic cancer. Is it any better. Arch Surg. 1995;130:838-42; discussion 842-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 84] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Yeo CJ, Barry MK, Sauter PK, Sostre S, Lillemoe KD, Pitt HA, Cameron JL. Erythromycin accelerates gastric emptying after pancreaticoduodenectomy. A prospective, randomized, placebo-controlled trial. Ann Surg. 1993;218:229-37; discussion 237-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 266] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 19. | Cameron JL, Pitt HA, Yeo CJ, Lillemoe KD, Kaufman HS, Coleman J. One hundred and forty-five consecutive pancreaticoduodenectomies without mortality. Ann Surg. 1993;217:430-45; discussion 430-45;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 565] [Article Influence: 17.7] [Reference Citation Analysis (33)] |
| 20. | Tanaka M, Sarr MG. Total duodenectomy: effect on canine gastrointestinal motility. J Surg Res. 1987;42:483-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Hocking MP, Harrison WD, Sninsky CA. Gastric dysrhythmias following pylorus-preserving pancreaticoduodenectomy. Possible mechanism for early delayed gastric emptying. Dig Dis Sci. 1990;35:1226-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Liberski SM, Koch KL, Atnip RG, Stern RM. Ischemic gastroparesis: resolution after revascularization. Gastroenterology. 1990;99:252-257. [PubMed] |
| 23. | Traverso LW, Longmire WP. Preservation of the pylorus in pancreaticoduodenectomy a follow-up evaluation. Ann Surg. 1980;192:306-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 151] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Mitchell GA. A macroscopic study of the nerve supply of the stomach. J Anat. 1940;75:50-63. [PubMed] |
| 26. | Skandalakis JE, Gray SW, Soria RE, Sorg JL, Rowe JS. Distribution of the vagus nerve to the stomach. Am Surg. 1980;46:130-139. [PubMed] |
| 27. | Skandalakis LJ, Gray SW, Skandalakis JE. The history and surgical anatomy of the vagus nerve. Surg Gynecol Obstet. 1986;162:75-85. [PubMed] |
| 28. | Yi SQ, Shimokawa T, Akita K, Ohta T, Kayahara M, Miwa K, Tanaka S. Anatomical study of the pancreas in the house musk shrew (Suncus murinus), with special reference to the blood supply and innervation. Anat Rec A Discov Mol Cell Evol Biol. 2003;273:630-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Yi SQ, Miwa K, Ohta T, Kayahara M, Kitagawa H, Tanaka A, Shimokawa T, Akita K, Tanaka S. Innervation of the pancreas from the perspective of perineural invasion of pancreatic cancer. Pancreas. 2003;27:225-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 89] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 30. | Yi SQ, Ohta T, Miwa K, Shimokawa T, Akita K, Itoh M, Miyamoto K, Tanaka S. Surgical anatomy of the innervation of the major duodenal papilla in human and Suncus murinus, from the perspective of preserving innervation in organ-saving procedures. Pancreas. 2005;30:211-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Yi SQ, Akita K, Ohta T, Shimokawa T, Tanaka A, Ru F, Nakatani T, Isomura G, Tanaka S. Cellular localization of endocrine cells in the adult pancreas of the house musk shrew, Suncus murinus: a comparative immunocytochemical study. Gen Comp Endocrinol. 2004;136:162-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | Wind P, Chevallier JM, Sarcy JJ, Delmas V, Cugnenc PH. The infrapyloric artery and cephalic pancreatoduodenectomy with pylorus preservation: preliminary study. Surg Radiol Anat. 1994;16:165-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 33. | Grace PA, Pitt HA, Longmire WP. Pylorus preserving pancreatoduodenectomy: an overview. Br J Surg. 1990;77:968-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 93] [Article Influence: 2.7] [Reference Citation Analysis (0)] |