Published online Mar 21, 2006. doi: 10.3748/wjg.v12.i11.1813
Revised: July 20, 2005
Accepted: August 26, 2005
Published online: March 21, 2006
We described two members in a family with gastroin-testinal stromal tumors (GISTs) without cutaneous hyperpigmentation. The patients were father and son who did not have cutaneous hyperpigmentation. Histological examination showed that these tumors were GISTs expressing CD34 and CD117. Tumor DNA extracted from paraffin-embedded specimens revealed somatic mutation with a deletion mutation at different codons in exon 11 of c-kit gene after direct sequencing analysis. No germline mutation was detected in DNA extracted from peripheral leukocytes obtained from the father and son. We propose that GISTs could be caused by sporadic somatic mutation in a family without germline mutation and hyperpigmentation.
- Citation: Yeh CN, Chen TW, Jan YY. Sporadic somatic mutation of c-kit gene in a family with gastrointestinal stromal tumors without cutaneous hyperpigmentation. World J Gastroenterol 2006; 12(11): 1813-1815
- URL: https://www.wjgnet.com/1007-9327/full/v12/i11/1813.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i11.1813
Gastrointestinal stromal tumor (GIST) is the most common mesenchymal tumor of the human gastrointestinal (GI) tract, representing 0.1 to 3 % of all GI tract tumors[1]. It has been suggested that a mutation in the juxtamembrane (JM) domain of c-kit contributes to the development of GIST[2]. Furthermore, germline deletion or point mutation of the c-kit JM domain has been shown in a family with GIST and cutaneous hyperpigmentation[3,4]. GIST-cutaneous hyperpigmentation disease has been used to describe familial multiple GISTs with associated cutaneous hyperpigmentation[4]. Familial GIST is a rare autosomal dominant genetic disorder associated with kit germline mutations. We now report that GISTs could be caused by sporadic somatic mutation in a family without germline mutation and cutaneous hyperpigmentation.
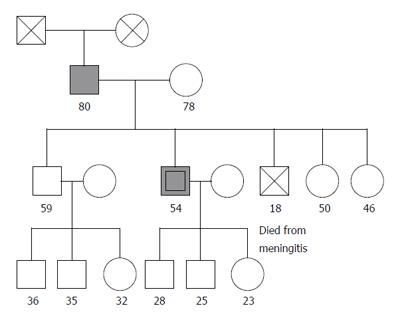
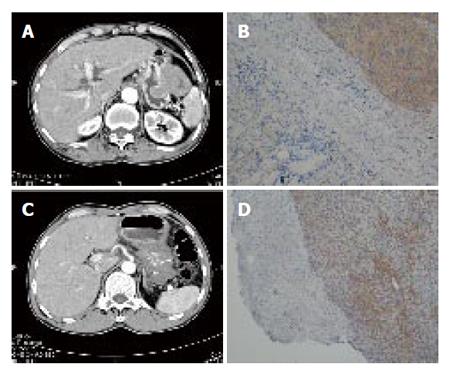
A 79-year-old man was referred to our hospital because of a gastric tumor (Figure 1). He complained of epigastria pain and fullness. There was no cutaneous hyperpigmentation. Gastroduodenal endoscopic examination and endoscopic ultrasonography (EUS) showed a gastric submucosal tumor (SMT) about 1.7 cm in size with small ulcer located at anterior wall, greater curvature side of high body. Proton pump inhibitor therapy was administered for 10 months. The SMT enlarged to 5cm in size demonstrated by upper gastrointestinal (GI) series, endoscopic ultrasonography (EUS), and abdominal computed tomography (CT) (Figure 2A). Wedge resection of the gastric SMT at the anterior wall, greater curvature side of high body with spleen preservation was performed with clear resected margin. Histological and immunohistochemical examination revealed low risk gastrointestinal stromal tumor (GIST) with strong positivity of c-kit and CD34 (Figure 2B). The patient lived free of disease for 9 months after surgery.
A 54-year-old man, the son of the patient 1, was referred to our hospital because of a gastric tumor (Figure 1). He complained of epigastria pain, abdominal fullness, body weight loss 7 to 8 kilograms for one month. There was no cutaneous hyperpigmentation. Gastroduodenal endoscopic examination and EUS showed a huge gastric SMT about 10 cm in size located at greater curvature side from high body to antrum with a deep ulcer and easily touch bleeding. Subsequent upper GI series and abdominal CT revealed a gastric SMT about 13×10 cm in size occupying the whole stomach (Figure 2C). Exploratory laparotomy revealed advanced gastric GIST with major vessel involvement. Biopsy of the gastric SMT and feeding jejunostomy was performed. Histological and immunohistochemical examination revealed advanced high-risk GIST with strong positivity of c-kit and CD34 (Figure 2D). The patient received 400 mg Glivec for 7 months and partial response was illustrated by subsequent abdominal CT. The patient lived with disease for 7 months after surgery.
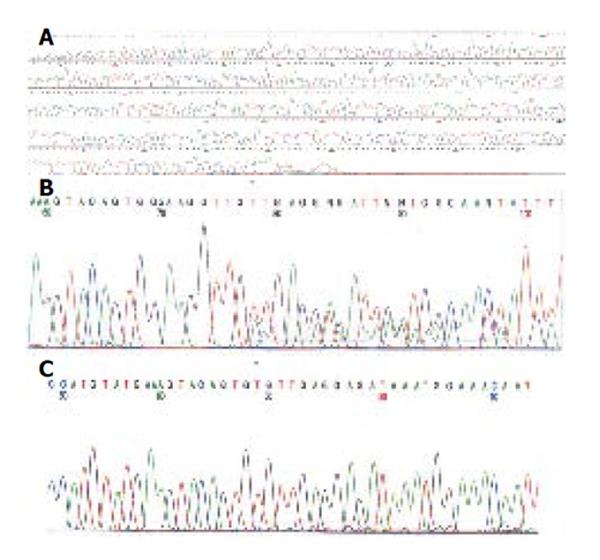
To determine whether these GISTs showed a genetic defect, DNA was extracted from paraffin-embedded specimen of tumor tissues. Polymerase chain reaction amplification of genomic DNA for kit and PDGFRA was performed and amplification was analyzed for mutations as previously described[5]. Direct sequencing analysis of DNA from patient 1 showed deletion mutation at codon 560 in exon 11, causing a deletion mutant 560 del V (Figure 3B). While direct sequencing analysis of DNA from patient 2 revealed deletion at codons 557-559 in exon 11, resulting in replacement of WKV by C (Figure 3C). To determine if the mutation is familial, DNA was extracted from peri-pheral leukocytes obtained from patients 1 and 2. No mutation was detected in exon 11 of c-kit gene (Figure 3A).
By histological, immunohistochemical examination, and molecular genetic analysis, this study has uncovered sporadic c-kit somatic mutation in a family with GIST without cutaneous hyperpigmentation.
GISTs appear to be related the interstitial cells of Cajal of the mesenteric plexus[7]. These cells are considered as GI pacemaker cells, from the interface between the automatic innervation of the bowel wall and its smooth muscle[8,9]. GISTs express the cell-surface transmembrane receptor c-kit with a tyrosin kinase activity and kit onco-protein. There are frequent gain-of-function mutations of c-kit in GISTs. These mutations result in constitutive activation of kit signaling, which leads to uncontrolled cell proliferation and resistance to apoptosis. It has been recently reported that kit activation occurs in all cases of GISTs, regardless of the mutation status of kit. Most GISTs express constitutively activated mutant isoforms of kit kinase or platelet-derived growth factor receptor alpha (PDGFRA), which are potential therapeutic targets for Imatinib mesylate (Glivec).
Gain of function mutations in the JM domain of c-kit contribute to the development of GIST[2]. Because normal kit gene is responsible to normal pigmentation, relationships between GISTs and cutaneous hyperpigmentation have been reported before. GIST-cutaneous hyperpigmentation disease has been used to describe familial multiple GISTs associated with cutaneous hyperpigmentation[3]. Furthermore, germline deletion mutation of the c-kit JM domain has been shown in tumors and normal somatic cells from a family with multiple GISTs who exhibited perineal hyperpigmentation[3]. A single-point germline mutation of c-kit has also been proposed to cause a familial GIST associated with systemic cutaneous hyperpigmentation[4]. A germline PDGRF missense mutation could be a second familial predisposing gene[5].
We described a rare case report regarding two members in a family with GISTs without cutaneous hyperpigmentation. No mutation was detected in DNA extracted from peripheral leukocytes obtained from the father and son. DNA extracted from paraffin-embedded specimens revealed different somatic mutation with a deletion mutation in the exon 11 of c-kit gene after direct sequencing analysis (deletion mutation at codon 560 versus deletion at codons 557-559 in exon 11). So mutation in the tumor is sporadic somatic but not germline. However, the cause of sporadic c-kit mutation in one family is unknown.
In summary, we propose that GISTs could be caused by sporadic somatic mutation in a family without germline mutation and hyperpigmentation.
This study appreciated Novartis (Taiwan) Co., Ltd for financial support of genetic analysis.
S- Editor Guo SY L- Editor Zhang JZ E- Editor Ma WH
| 1. | Lewis JJ, Brennan MF. The management of retroperitoneal soft tissue sarcoma. Adv Surg. 1999;33:329-344. [PubMed] |
| 2. | Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3215] [Cited by in RCA: 3115] [Article Influence: 115.4] [Reference Citation Analysis (0)] |
| 3. | Nishida T, Hirota S, Taniguchi M, Hashimoto K, Isozaki K, Nakamura H, Kanakura Y, Tanaka T, Takabayashi A, Matsuda H. Familial gastrointestinal stromal tumours with germline mutation of the KIT gene. Nat Genet. 1998;19:323-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 388] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 4. | Maeyama H, Hidaka E, Ota H, Minami S, Kajiyama M, Kuraishi A, Mori H, Matsuda Y, Wada S, Sodeyama H. Familial gastrointestinal stromal tumor with hyperpigmentation: association with a germline mutation of the c-kit gene. Gastroenterology. 2001;120:210-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 159] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 5. | Chompret A, Kannengiesser C, Barrois M, Terrier P, Dahan P, Tursz T, Lenoir GM, Bressac-De Paillerets B. PDGFRA germline mutation in a family with multiple cases of gastrointestinal stromal tumor. Gastroenterology. 2004;126:318-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 165] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 6. | Heinrich MC, Corless CL, Demetri GD, Blanke CD, von Mehren M, Joensuu H, McGreevey LS, Chen CJ, Van den Abbeele AD, Druker BJ. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21:4342-4349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1817] [Cited by in RCA: 1647] [Article Influence: 74.9] [Reference Citation Analysis (1)] |
| 7. | Akwari OE, Dozois RR, Weiland LH, Beahrs OH. Leiomyosarcoma of the small and large bowel. Cancer. 1978;42:1375-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 8. | Shiu MH, Farr GH, Papachristou DN, Hajdu SI. Myosarcomas of the stomach: natural history, prognostic factors and management. Cancer. 1982;49:177-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 9. | McGrath PC, Neifeld JP, Lawrence W Jr, Kay S, Horsley JS 3rd, Parker GA. Gastrointestinal sarcomas. Analysis of prognostic factors. Ann Surg. 1987;206:706-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 138] [Article Influence: 3.6] [Reference Citation Analysis (0)] |