Published online Mar 21, 2006. doi: 10.3748/wjg.v12.i11.1723
Revised: October 21, 2005
Accepted: November 18, 2005
Published online: March 21, 2006
AIM: To determine by brain functional magnetic resonance imaging (fMRI) whether cerebral processing of non-visceral stimuli is altered in irritable bowel syndrome (IBS) patients compared with healthy subjects. To circumvent spinal viscerosomatic convergence mechanisms, we used auditory stimulation, and to identify a possible influence of psychological factors the stimuli differed in their emotional quality.
METHODS: In 8 IBS patients and 8 controls, fMRI measurements were performed using a block design of 4 auditory stimuli of different emotional quality (pleasant sounds of chimes, unpleasant peep (2000 Hz), neutral words, and emotional words). A gradient echo T2*-weighted sequence was used for the functional scans. Statistical maps were constructed using the general
linear model.
RESULTS: To emotional auditory stimuli, IBS patients relative to controls responded with stronger deactivations in a greater variety of emotional processing regions, while the response patterns, unlike in controls, did not differentiate between distressing or pleasant sounds. To neutral auditory stimuli, by contrast, only IBS patients responded with large significant activations.
CONCLUSION: Altered cerebral response patterns to auditory stimuli in emotional stimulus-processing regions suggest that altered sensory processing in IBS may not be specific for visceral sensation, but might reflect
generalized changes in emotional sensitivity and affective reactivity, possibly associated with the psychological comorbidity often found in IBS patients.
- Citation: Andresen V, Poellinger A, Tsrouya C, Bach D, Stroh A, Foerschler A, Georgiewa P, Schmidtmann M, Voort IRVD, Kobelt P, Zimmer C, Wiedenmann B, Klapp BF, Monnikes H. Cerebral processing of auditory stimuli in patients with irritable bowel syndrome. World J Gastroenterol 2006; 12(11): 1723-1729
- URL: https://www.wjgnet.com/1007-9327/full/v12/i11/1723.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i11.1723
Visceral hypersensitivity has been shown to play a patho-genic role in the irritable bowel syndrome (IBS)[1,2]. At least in a subgroup of patients, this might be caused by altered brain processing of visceral sensation as has been suggested on the basis of brain imaging studies. Another important characteristic of IBS is the extraintestinal comorbidity. There is a high prevalence of non-gastrointes-tinal functional diseases, such as fibromyalgia or chronic fatigue syndrome, and also psychological disorders, such as depression and anxiety[3,4]. The frequent co-occurrence of these different disorders on the one hand, and the impact of psychological factors on all of them on the other hand[5], leads to the question whether these disorders might share a common pathogenesis such as a generalized increase in emotional and sensory sensitivity that could be involved in the alterations of sensory brain processing observed in these disorders[6-9].
Indeed, there is some evidence that altered cerebral response patterns in IBS may not be specific for gastr-ointestinal stimuli. In patients with IBS and concomitant fibromyalgia, alterations in the central pro-cessing of painful somatic stimuli have been observed[7]. Moreover, even IBS patients without a manifest fibr-omyalgia may exhibit a somatic hypersensitivity with altered cerebral processing of somatic sensation[10]. These forms of somatic hypersensitivity, which are in contrast to earlier descriptions of somatic hyposensitivity states in IBS, are caused by viscerosomatic convergence mechanisms. The latter are thought to occur, because visceral and somatic sensations are both passed through the dorsal root ganglia and the dorsal columns of the spinal cord, where neural interactions have been described[11]. Therefore,any peripherally generated visceral hypersensitivity could subsequently induce a somatic hypersensitivity in the co-rresponding somatic dermatoma, and altered cerebral response pattern to somatic stimuli would actually reflect increased sensory input from the periphery. However, further support for cerebrally located processing alter-ations in IBS is provided by two studies demonstrating an increased reactivity of event-related potentials to auditory stimuli in IBS patients[12,13]. Since auditory stimuli are transmitted directly to the brain via the eighth cranial nerve, there is no peripheral connection to visceral sensory input and changes in the central reactivity could be allo-cated directly to the sensory processing circuits of the brain.
With this study, we aimed to test the hypothesis that IBS patients differ from healthy controls in the cerebral processing of non-visceral stimuli, detected by functional magnetic resonance imaging (fMRI). To be able to focus on the cerebral processing level, we chose auditory cues as non-visceral stimuli. The specific aims were 1) to analyze by fMRI the cerebral response patterns to non-visceral, auditory stimuli in IBS patients compared with healthy controls, and 2) to evaluate whether different brain responses are influenced by the emotional impact of the stimulus.
Eight right-handed patients with IBS [five females, three males; mean age, 41.3 (27-64) years; diarrhea-predominant, n =5, alternating stools, n =3] established by Rome II criteria [14] were recruited at our institution’s outpatient clinic for gastrointestinal functional disorders. To exclude other causes for bowel symptoms, all patients had undergone a thorough work-up including laboratory tests, stool analysis for bacterial, fungal, or parasite infection, abdominal ultrasound, colonoscopy, lactose- and fructose hydrogen breath test. Moreover, a rectal sensitivity testing by computerized barostat was performed. The mean individual perception threshold of first sensation of the rectal barostat distension was 16.4 mmHg (SD 6.2). None of the patients had previous abdominal surgery and all patients had symptoms for more than 1 year. None of the patients used centrally acting agents to treat bowel symptoms, and peripherally acting IBS treatments were stopped 7 d prior to the study. As controls, 8 right-handed healthy volunteers [5 males, 3 females; mean age, 39.4 (24-54) years] were selected after exclusion of individuals with concomitant or previous GI-disorders by history and the IBS symptom score-questionnaire [15]. In all subjects, written informed consent as approved by the institutional ethical committee was obtained, and concomitant psychiatric disorders were excluded by using the standardized German Diagnostic Interview for Psychiatric Disorders (DIPS[16]), a structured clinical interview based on the Anxiety Disorders Interview Schedule[17].
For the assessment of personality characteristics, all study
participants were asked to fill in the following questionn-aires: The German form of the State-Trait-Anxiety-Inventory (STAI)[18] for the assessment of depression, neuroticism and complaints, the German form of the Beck’s Depression Index (BDI)[19] for the assessment of depression, and the German form of the NEO-Five-Factor-Inventory (NEO-FFI)[20] for the assessment of neuroticism, openness to experience, extroversion, agreeableness, and conscientiousness.
We used a block design of four auditory stimuli of diff-erent emotional impact: an unpleasant peep (a sound of 2000 Hz), pleasant sounds of chimes, words with emotional impact and neutral words. The part of the protocol using neutral words had served as a neutral, non-visceral control stimulus, in a previous study investigating visceral stimulation[21]. All words were chosen from a list of words that had been evaluated as neutral or emotional by a group of 20 healthy volunteers. The stimulation paradigm had been evaluated in event-related potential studies for patients with psychosomatic disorders. The stimulation phases of 48 s each were separated by resting phases and applied in the following order: peep, chimes, neutral words, chimes, emotional words, neutral words, peep.
Magnetic resonance images were collected on a 1.5 T whole
body scanner (Siemens Magnetom Vision, Erlangen, Germ-
any) with a standard head coil. A vacuum pad was used to minimize head movements. First, a T1-weighted localizer scan was recorded. Next, T2-weighted oblique scans were obtained (TR/TE 4500/128 ms, field of view 230 mm), primarily to aid Talairach transformation for data analysis. For the functional scans, an echo-planar sequence (TR/TE 4000/66 ms; flip angle 90 degrees; field of view 230 mm; matrix 128 × 128; slice thickness 6 mm, interslice gap 0.6 mm; in-plane resolution 1.8 mm × 1.8 mm) was used. One hundred and twenty images per slice were acquired. Sixteen slices adjusted at a transverse-to-coronal angle of approximately 20° covering the whole brain with the exception of the most superior frontal and superior parietal lobe, inferior temporal pole, and cerebellum (most superior z about 60 and most inferior z about -25 according to Talairach and Tournoux[22]) were obtained for all studies. Structural 3D data sets were acquired using a T1-weighted sagittal sequence with isotropic voxels (TR/TE 11.4/4.4 ms; flip angle 15 degrees; number of slices 160, matrix 256 × 256, field of view 256 mm, voxel size 1 mm3).
For data analysis we used the Brainvoyager® software (Brain Innovation B.V, Postbus, Maastricht, Netherlands). The 2D functional data were reconstructed within the 3D structural data set. The 3D-data were then transformed into the standardized Talairach spaced brain[22]. Finally, the reconstructed data set underwent subsequent procedures of motion correction, intensity scaling and detrending.
Statistical maps were constructed using the general linear model module of the Brainvoyager software. The stimulation conditions were used as predictors, and the contrasts of each stimulation condition versus rest were analyzed. For the between group comparison, predictors were defined for each stimulus type as the interaction of the group with this stimulus type. Therefore, each predictor represented a larger signal increase of one group at the referred stimulus type.
Activated clusters were only accepted if they showed highly significant (P < 0.001) activation increase. In order to correct for multiple comparisons, a minimal cluster size of 6 voxels was defined.
As regions of interest (RoI) for the data analysis (Table 2), we selected brain areas known to be involved in emotional processing[23]. All regions were neuroanatomically pre-defined by an expert neuroradiologist according to the coo-rdinates of Talairach et al[22], and according to neuro-anatomical visualization.
| I) PEEP | 1Largest cluster | ||||||
| A: Control deactivation | |||||||
| RoI | Side | No. of | No. of | x1 | y1 | z1 | % signal |
| Clusters | Voxels1 | change* | |||||
| ACC | L | 1 | -793 | -8 | 37 | -10 | -0.53 |
| PFC | B | 7 | -1506 | 37 | 35 | -7 | -0.52 |
| B: IBS deactivation | |||||||
| ACC | L | 1 | -271 | -6 | 31 | 7 | -0.28 |
| PFC | B | 7 | -1724 | 23 | 57 | 8 | -0.52 |
| Amyg - dala | R | 1 | -452 | 25 | 1 | -20 | -0.33 |
| HC | B | 2 | -436 | 33 | -22 | -24 | -0.38 |
| II) CHIMES | |||||||
| A: Control activation | |||||||
| ACC | B | 1 | 699 | -1 | 29 | 2 | 0.40 |
| PFC | B | 7 | 1957 | -4 | 37 | -6 | 0.39 |
| Deactivation | |||||||
| PFC | B | 4 | -1781 | 31 | 47 | 23 | -0.48 |
| PCC | L | 1 | -707 | -5 | -4 | 51 | -0.24 |
| Insula | R | 1 | -850 | 32 | 1 | 7 | -0.25 |
| HC | R | 1 | -113 | 26 | -14 | -15 | -0.14 |
| B: IBS deactivation | |||||||
| ACC | B | 2 | -1212 | 2 | 18 | 44 | -0.38 |
| PCC | B | 1 | -159 | 0 | -17 | 32 | -0.36 |
| PFC | R | 3 | -2095 | 54 | 13 | 13 | -0.42 |
| III) EMOTIONAL WORDS | 1Largest cluster | ||||||
| A: Control deactivation | |||||||
| ACC | L | 1 | -724 | -5 | 36 | 13 | -0.33 |
| PFC | B | 4 | -275 | -4 | 47 | -2 | -0.36 |
| PCC | L | 1 | -134 | -1 | -33 | 44 | -0.35 |
| PSAC | B | 1 | -2048 | 0 | -62 | 33 | -0.36 |
| HC | R | 1 | -31 | 25 | -24 | -15 | -0.21 |
| B: IBS activation | |||||||
| PFC | L | 1 | 609 | -46 | 29 | -1 | 0.25 |
| Deactivation | |||||||
| ACC | R | 1 | -1207 | 2 | 40 | 5 | -0.41 |
| PFC | B | 4 | -2076 | 9 | 43 | 26 | -0.32 |
| PSAC | B | 1 | -2048 | 0 | -46 | 42 | -0.35 |
| HC | B | 2 | -1010 | 24 | -35 | -12 | -0.29 |
| Insula | R | 1 | -331 | 34 | -10 | 1 | -0.19 |
| IV) NEUTRAL WORDS | |||||||
| A : Controls activation | |||||||
| PFC | R | 1 | 45 | 26 | 55 | 6 | 0.40 |
| PSAC | R | 1 | 45 | 26 | -42 | 53 | 0.05 |
| Deactivation | |||||||
| ACC | L | 1 | -118 | -3 | 31 | -6 | -0.44 |
| B : IBS activation | |||||||
| ACC | R | 2 | 223 | 1 | 6 | 46 | 0.19 |
| PFC | B | 8 | 1408 | 0 | 46 | -11 | 0.18 |
| PSAC | B | 4 | 257 | 26 | -65 | 41 | 0.08 |
| HC | B | 2 | 652 | 27 | -27 | 19 | 0.14 |
| PCC | L | 2 | 102 | -2 | -39 | 37 | 0.12 |
The personality traits were not significantly different between IBS patients and healthy controls. Results are summ-arized in Table 1. Descriptively, IBS patients had higher values for anxiety (P = 0.17), depression (P = 0.19) and neuroticism (P = 0.11) relative to healthy controls (Table 1).
| Personality test | Control | IBS | IBS vs control | Reference- population [18-20] |
| STAI X2 | 34.8 ± 6.1 | 41.3 ± 11.0 | P = 0.17 | 35.7 ± 9.4 |
| BDI | 5.4 ± 5.3 | 8.7 ± 4.4 | P = 0.19 | 6.5 ± 5.2 |
| NEO: Neuroticism | 1.7 ± 0.5 | 2.2 ± 0.6 | P = 0.11 | 1.8 ± 0.7 |
| NEO: Extroversion | 2.8 ± 0.4 | 2.6 ± 0.3 | P = 0.24 | 2.4 ± 0.6 |
| NEO: Openness to experience | 2.5 ± 0.5 | 2.4 ± 0.3 | P = 0.73 | 2.7 ± 0.5 |
| NEO: Agreeableness | 2.4 ± 0.6 | 2.4 ± 0.3 | P = 0.81 | 2.4 ± 0.5 |
| NEO: Consciousness | 3.0 ± 0.6 | 3.2 ± 0.5 | P = 0.46 | 2.6 ± 0.6 |
Both groups responded to all stimuli with a significant activation (P < 0.001) in the auditory cortex, with a high-er signal response to the word processing stimulus co-nditions and in the speech processing areas for the word stimulations (data not shown). With regard to the emo-tional processing regions we observed the following:
To unpleasant peep, both groups responded with sig-nificant deactivations (P < 0.001) in the anterior cingulate cortex (ACC) and prefrontal cortex (PFC) with a relatively stronger ACC deactivation in controls, as indicated by the number of deactivated voxels. By contrast, only IBS patients responded with significant deactivations (P < 0.001) in the amygdala and the hippocampus (HC) (Table 2).
To pleasant sounds of chimes, both groups responded with significant deactivations (P < 0.001) in the PFC and the posterior cingulated cortex (PCC) with a relatively stronger PCC deactivation in controls, as indicated by the number of deactivated voxels. Only controls responded with deactivations (P < 0.001) in the Insula and HC, and with significant activations (P < 0.001) in the ACC and a different region of the PFC. In IBS patients, on the contrary, the ACC was significantly deactivated (P < 0.001) (Table 2).
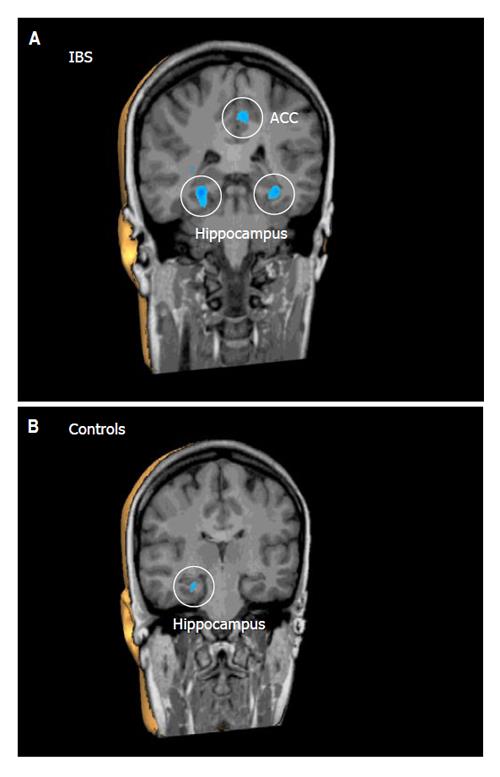
To emotional words, both groups responded with significant deactivations (P < 0.001) in the parietal sensory association cortex (PSAC), the ACC, the PFC, and the HC. However, the overall deactivation response in the ACC, PFC and HC, as indicated by number and size of deactivated clusters, was much stronger in IBS patients relative to controls (Table 2, Figure 1). By contrast, only controls responded with a significant deactivation (P < 0.001) in the PCC, while only in IBS patients, emotional words induced deactivations (P < 0.001) in the insula and moreover a significant activation (P < 0.001) in a different region of the PFC (Table 2).
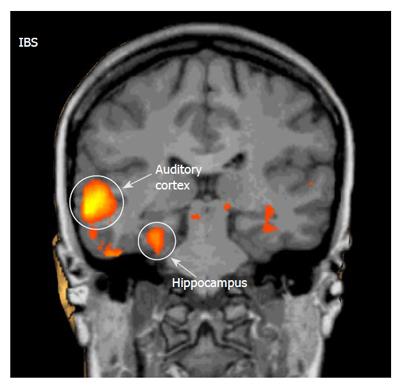
To neutral words, both groups responded with significant activations (P < 0.001) in the PFC and PSAC. However, the overall activation response, as indicated by number and size of activated clusters, was much stronger in IBS patients relative to controls. Only in controls, neutral words induced a deactivation (P < 0.001) in the ACC. By contrast, only IBS patients responded with significant activations (P < 0.001) in the ACC, PCC, HC, and insula (Table 2, Figure 2).
Altered cerebral processing of visceral sensation has been proposed to play a pathogenic role in IBS. Whether these alterations are specific for visceral stimuli is currently not clear. In this study, we analyzed cerebral processing of non-visceral, auditory stimuli using fMRI. In a separate protocol, all IBS patients included in this study also underwent rectal stimulation by balloon distension adapted to the individual rectal perception thresholds, which were lower in IBS patients than in controls[21]. In IBS patients, decreased ACC and PFC activation with subliminal and supraliminal rectal stimuli and increased HC activation with supraliminal stimuli suggested disturbances of the associative and emotional processing of visceral sensation[21]. Our current findings demonstrate that IBS patients exhibit differences in the central processing of auditory stimuli of different emotional impact in brain regions involved in emotional sensory processing. Our main observations were: 1) To distressing auditory stimuli (peep and emotional words) both controls and IBS patients responded with significant deactivations in emotional pro-cessing regions demonstrating stronger responses in a gr-eater variety of brain areas in IBS patients. 2) To pleasant auditory stimuli (chimes) IBS patients also responded with significant deactivations only, while in controls significant activations in the ACC and PFC were observed. 3) To neutral auditory stimuli (neutral words) IBS patients, unlike controls, responded with large significant activations in a variety of emotional processing regions. Taken together, IBS patients reacted more intensively with deactivations following emotional auditory stimuli, not differentiating, unlike controls, between distressing or pleasant sounds, and more intensively reacted with activations to neutral auditory stimuli.
Thus, this fMRI study indicates alterations of auditory sensory processing in IBS patients in brain regions participating in emotional stimulus processing. The cerebral processing differences involve both activation and deactivation responses. However, the specific cerebral function of deactivations observed in fMRI is still not completely understood and has been widely discussed[24]. Within the context of BOLD (Blood Oxygen Level Dependent) responses, on which the fMRI technique is based on, an activation reflects an increase in regional oxyhemoglobin that is believed to result from an increased blood flow coupled to neural activation (“neurovasular coupling”). In contrast, a “deactivation” reflects a stimulation-induced regional decrease of oxyhemoglobin of which the functional meaning is unclear. Vascular steal mechanisms or “neurovascular decoupling” resulting in reduced oxyhemoglobin have been suggested as possible cause of signal decrease[24]. In this case, the signal decrease would also reflect a neural activation. On the other hand, there is some evidence that the signal decrease could actually reflect a reduction of neural activity[25-27]. In fact, it has been proposed, that deactivations might contribute to cortical protection from incoming stimuli, for example in the context of chronic repetitive subconscious somatic stimuli[28] or in the context of sleep preservation[26,27]. A study demonstrating that the same auditory stimulus can induce either activation or deactivation responses of the amygdala just depending on the psychosocial background of the subjects[29],also supports a distinct neuro-functional meaning of deactiva-tions. However, the uncertain functional meaning of de-activations to date makes the detailed interpretation of our findings difficult and we therefore restrict the following discussion to a rather descriptive evaluation of the dem-onstrated brain processing differences.
Though both IBS patients and controls responded to distressing emotional stimuli mainly with deactivations, the deactivated regions in IBS patients were larger and involved a greater variety of emotional processing regions, among them parts of the limbic system like the amygdala or the hippocampus. In general, these observations could suggest a greater emotional reactivity to distressing stimuli in IBS patients as compared with healthy controls. This is in line with previous literature that demonstrate a central hyperreactivity of IBS patients to rectal stimuli in the presence of distressing auditory or visual stimuli[30-32].
Interestingly, IBS patients responded to pleasant sounds with similar deactivation patterns as to distressing stimuli whereas controls also reacted with activations. It is conceivable that an underlying alexithymia, a disorder characterized by the difficulty to recognize or express emotions and found to be significantly increased in IBS patients[33], could possibly accord for this finding. However, this has to remain speculative, because alexithymia was not specifically assessed in this study.
Neutral words, as expected, did hardly induce any reac-tion in emotional processing centers in healthy controls. By contrast, IBS patients responded with significant activations in these brain regions. These findings could indicate that IBS patients have a generalized increase in the sensitivity and reactivity of emotional processing brain regions even in response to neutral stimuli. Interestingly, the reaction to neutral stimuli is limited to activation-responses, while the emotional stimuli induced deactivation patterns in the same brain regions. This provides further evidence that activations and deactivations represent different processing functions depending on the emotional impact of the stimulus[29].
Overall, our findings indicate that IBS patients relative to controls show a greater cerebral reactivity to auditory stimuli with a larger involvement of limbic structures suggesting a higher sensitivity of emotional processing brain regions. Thereby, our results underline earlier observations by Blomhoff et al and Berman et al[12,13,34] who demonstrated a cerebral hyperreactivity to both emotional and neutral auditory stimuli in IBS patients. While al-tered cerebral processing in IBS has previously been de-monstrated in the context of visceral stimulation[6,35,36], the current findings suggest that differences in cerebral stimulus processing in IBS might not be restricted to visceral sensation. Rather it is conceivable that differences in the emotional state might be an underlying factor. In fact, Blomhoff could demonstrate that the hyperreactivity to auditory stimuli is especially found in IBS patients with concomitant phobic anxiety disorders[34]. Accordingly, in our study, higher levels of anxiety, depression, and neuroticism in the IBS patient group (though differences were not reaching the level if significance possibly due to small sample sizes) could account for some of the observed differences in brain activation responses.
We acknowledge that this study has several limitations: The sample size of n = 8 for each group is rather small, and the bowel dysfunctions are heterogeneous in the IBS group. There is some evidence that diarrhea- versus constipation-predominant IBS patients have different cere-bral response patterns to rectal stimulation[37]. Whether this could also affect the cerebral processing of auditory stimuli is unknown. Furthermore, it is conceivable that the increased scores for anxiety, neuroticism and depression can alone account for the different stimulus processing of patients versus controls independent of IBS status. Future studies should include more patients, compare also to other psychologically distressed patients free of IBS, assess further psychometric variables such as alexithymia and allow for different subgroup analyses to further elucidate the nature of altered sensory processing in IBS.
Overall, the current observations of altered cerebral response patterns to neutral and emotional auditory stimuli
in IBS patients indicate that altered emotional stimulus processing in IBS may not be specific for visceral sensation, but might reflect a generalized increase in emotional sensitivity and affective reactivity. This could account for the frequent association of IBS with psychological or extra-intestinal functional disorders[3].
We thank Norbert Brombacher and Mathias Moosman, Department of Neurology, Charite - Universitätsmedizin Berlin, for their advice and support.
S- Editor Pan BR L- Editor Zhang JZ E- Editor Wu M
| 1. | Mertz H, Naliboff B, Munakata J, Niazi N, Mayer EA. Altered rectal perception is a biological marker of patients with irritable bowel syndrome. Gastroenterology. 1995;109:40-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 679] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 2. | Bouin M, Plourde V, Boivin M, Riberdy M, Lupien F, Laganiere M, Verrier P, Poitras P. Rectal distention testing in patients with irritable bowel syndrome: sensitivity, specificity, and predictive values of pain sensory thresholds. Gastroenterology. 2002;122:1771-1777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 342] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 3. | Whitehead WE, Palsson O, Jones KR. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: what are the causes and implications. Gastroenterology. 2002;122:1140-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 775] [Cited by in RCA: 774] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 4. | Porcelli P. Psychological abnormalities in patients with irritable bowel syndrome. Indian J Gastroenterol. 2004;23:63-69. [PubMed] |
| 5. | Mayer EA, Naliboff BD, Chang L, Coutinho SV. V. Stress and irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2001;280:G519-G524. [PubMed] |
| 6. | Mertz H, Morgan V, Tanner G, Pickens D, Price R, Shyr Y, Kessler R. Regional cerebral activation in irritable bowel syndrome and control subjects with painful and nonpainful rectal distention. Gastroenterology. 2000;118:842-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 400] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 7. | Chang L, Berman S, Mayer EA, Suyenobu B, Derbyshire S, Naliboff B, Vogt B, FitzGerald L, Mandelkern MA. Brain responses to visceral and somatic stimuli in patients with irritable bowel syndrome with and without fibromyalgia. Am J Gastroenterol. 2003;98:1354-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 80] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Thomas KM, Drevets WC, Dahl RE, Ryan ND, Birmaher B, Eccard CH, Axelson D, Whalen PJ, Casey BJ. Amygdala response to fearful faces in anxious and depressed children. Arch Gen Psychiatry. 2001;58:1057-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 401] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 9. | Bramanti P, Grugno R, Vitetta A, Di Bella P, Muscarà N, Nappi G. Migraine with and without aura: electrophysiological and functional neuroimaging evidence. Funct Neurol. 2005;20:29-32. [PubMed] |
| 10. | Verne GN, Himes NC, Robinson ME, Gopinath KS, Briggs RW, Crosson B, Price DD. Central representation of visceral and cutaneous hypersensitivity in the irritable bowel syndrome. Pain. 2003;103:99-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 190] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 11. | Verne GN, Robinson ME, Price DD. Hypersensitivity to visceral and cutaneous pain in the irritable bowel syndrome. Pain. 2001;93:7-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 212] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 12. | Blomhoff S, Jacobsen MB, Spetalen S, Dahm A, Malt UF. Perceptual hyperreactivity to auditory stimuli in patients with irritable bowel syndrome. Scand J Gastroenterol. 2000;35:583-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Berman SM, Naliboff BD, Chang L, Fitzgerald L, Antolin T, Camplone A, Mayer EA. Enhanced preattentive central nervous system reactivity in irritable bowel syndrome. Am J Gastroenterol. 2002;97:2791-2797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Thompson WG, Longstreth GF, Drossman DA, Heaton KW, Irvine EJ, Müller-Lissner SA. Functional bowel disorders and functional abdominal pain. Gut. 1999;45 Suppl 2:II43-II47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 622] [Cited by in RCA: 830] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 15. | Heymann-Mönnikes I, Arnold R, Florin I, Herda C, Melfsen S, Mönnikes H. The combination of medical treatment plus multicomponent behavioral therapy is superior to medical treatment alone in the therapy of irritable bowel syndrome. Am J Gastroenterol. 2000;95:981-994. [PubMed] |
| 16. | Markgraf J, Schneider S, Ehlers A. Diagnostisches Interview bei psychischen Stoerungen (DIPS). Berlin: Springer. 1991;. |
| 17. | Brown , TA , DiNardo PA, Barlow DH. Anxiety disorders interview schedule ADIS-IV and ADIS-IV-L combination speciemn set. New York: Oxford University Press. 1993;. |
| 18. | Laux L, Glanzmann , P . Schaffner, P., Spiegelberger, C.D. Das State-Trait-Angstinventar (STAI). Weinheim: Beltz Testgesellschaft. 1981;. |
| 19. | Beck , AT , Hautzinger M, Bailer , M . Worall, H., Keller, F. Das Beck-Depressions-Inventar (BDI). 2nd ed. Bern: Huber. 1994;. |
| 20. | Borkenau P, Ostendorf , F . NEO-Fuenf-Faktoren Inventar (NEO-FFI). Goettingen, Bern, Toronto, Seattle, Goettingen, Bern, Toronto, Seattle: Hogrefe. 1993;. |
| 21. | Andresen V, Bach DR, Poellinger A, Tsrouya C, Stroh A, Foerschler A, Georgiewa P, Zimmer C, Mönnikes H. Brain activation responses to subliminal or supraliminal rectal stimuli and to auditory stimuli in irritable bowel syndrome. Neurogastroenterol Motil. 2005;17:827-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Talairach J, Tournoux P. Co-planar stereotactic atlas of the human brain. Stuttgart: Thieme. 1988;. [PubMed] |
| 23. | Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol Psychiatry. 2003;54:515-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1232] [Cited by in RCA: 1258] [Article Influence: 57.2] [Reference Citation Analysis (0)] |
| 24. | Wade AR. The negative BOLD signal unmasked. Neuron. 2002;36:993-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Moosmann M, Ritter P, Krastel I, Brink A, Thees S, Blankenburg F, Taskin B, Obrig H, Villringer A. Correlates of alpha rhythm in functional magnetic resonance imaging and near infrared spectroscopy. Neuroimage. 2003;20:145-158. [RCA] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 437] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 26. | Czisch M, Wehrle R, Kaufmann C, Wetter TC, Holsboer F, Pollmächer T, Auer DP. Functional MRI during sleep: BOLD signal decreases and their electrophysiological correlates. Eur J Neurosci. 2004;20:566-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 101] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Czisch M, Wetter TC, Kaufmann C, Pollmächer T, Holsboer F, Auer DP. Altered processing of acoustic stimuli during sleep: reduced auditory activation and visual deactivation detected by a combined fMRI/EEG study. Neuroimage. 2002;16:251-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 112] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 28. | Blankenburg F, Taskin B, Ruben J, Moosmann M, Ritter P, Curio G, Villringer A. Imperceptible stimuli and sensory processing impediment. Science. 2003;299:1864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Seifritz E, Esposito F, Neuhoff JG, Luthi A, Mustovic H, Dammann G, von Bardeleben U, Radue EW, Cirillo S, Tedeschi G. Differential sex-independent amygdala response to infant crying and laughing in parents versus nonparents. Biol Psychiatry. 2003;54:1367-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 242] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 30. | Phillips ML, Gregory LJ, Cullen S, Coen S, Ng V, Andrew C, Giampietro V, Bullmore E, Zelaya F, Amaro E. The effect of negative emotional context on neural and behavioural responses to oesophageal stimulation. Brain. 2003;126:669-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 143] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 31. | Morgan V, Pickens D, Gautam S, Kessler R, Mertz H. Amitriptyline reduces rectal pain related activation of the anterior cingulate cortex in patients with irritable bowel syndrome. Gut. 2005;54:601-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 202] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 32. | Blomhoff S, Spetalen S, Jacobsen MB, Vatn M, Malt UF. Intestinal reactivity to words with emotional content and brain information processing in irritable bowel syndrome. Dig Dis Sci. 2000;45:1160-1165. [RCA] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Portincasa P, Moschetta A, Baldassarre G, Altomare DF, Palasciano G. Pan-enteric dysmotility, impaired quality of life and alexithymia in a large group of patients meeting ROME II criteria for irritable bowel syndrome. World J Gastroenterol. 2003;9:2293-2299. [PubMed] |
| 34. | Blomhoff S, Spetalen S, Jacobsen MB, Malt UF. Phobic anxiety changes the function of brain-gut axis in irritable bowel syndrome. Psychosom Med. 2001;63:959-965. [PubMed] |
| 35. | Naliboff BD, Derbyshire SW, Munakata J, Berman S, Mandelkern M, Chang L, Mayer EA. Cerebral activation in patients with irritable bowel syndrome and control subjects during rectosigmoid stimulation. Psychosom Med. 2001;63:365-375. [PubMed] |
| 36. | Silverman DH, Munakata JA, Ennes H, Mandelkern MA, Hoh CK, Mayer EA. Regional cerebral activity in normal and pathological perception of visceral pain. Gastroenterology. 1997;112:64-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 359] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 37. | Wilder-Smith CH, Schindler D, Lovblad K, Redmond SM, Nirkko A. Brain functional magnetic resonance imaging of rectal pain and activation of endogenous inhibitory mechanisms in irritable bowel syndrome patient subgroups and healthy controls. Gut. 2004;53:1595-1601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 303] [Article Influence: 14.4] [Reference Citation Analysis (0)] |