Published online Mar 21, 2006. doi: 10.3748/wjg.v12.i11.1718
Revised: November 21, 2005
Accepted: December 7, 2005
Published online: March 21, 2006
AIM: To evaluate the effects of betaine on the ethanol-induced secretion of IGF-I and IGFBP-1 using radioimmunoassay and Western blotting, respectively, in primary cultured rat hepatocytes.
METHODS: Hepatocytes isolated from male Sprague-Dawley rats were incubated with various concentrations of ethanol and PD98059 procedures. The hepatocytes were also treated with different doses of betaine (10-5, 10-4, and 10-3 mol/L). We measured IGF-I and IGFBP-1 using radioimmunoassay and Western blotting, respectively.
RESULTS: The ethanol-induced inhibition of IGF-I secretion was attenuated by betaine in a concentration-dependent manner in primary cultured rat hepatocytes. At 10-3 mol/L, betaine significantly increased IGF-I secretion but decreased IGFBP-1 secretion. In addition, p42/44 mitogen-activated protein kinase (MAPK) activity was accelerated significantly from 10 min to 5 h after treatment with 10-3 mol/L betaine. Furthermore, the changes in IGF-1 and IGFBP-1 secretion resulting from the increased betaine-induced p42/44 MAPK activity in primary cultured rat hepatocytes was blocked by treatment with the MAPK inhibitor PD98059. Betaine treatment blocked the ethanol-induced inhibition of IGF-I secretion and p42/44 MAPK activity, and the ethanol-induced increase in IGFBP-1 secretion.
CONCLUSION: Betaine modulates the secretion of IGF-I and IGFBP-1 via the activation of p42/44 MAPK in primary cultured rat hepatocytes. Betaine also alters the MAPK activations induced by ethanol.
- Citation: Lee MS, Kim MS, Park SY, Kang CW. Effects of betaine on ethanol-stimulated secretion of IGF-I and IGFBP-1 in rat primary hepatocytes: Involvement of p42/44 MAPK activation. World J Gastroenterol 2006; 12(11): 1718-1722
- URL: https://www.wjgnet.com/1007-9327/full/v12/i11/1718.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i11.1718
Betaine, an organic osmolyte, is a precursor of S-adenosylmethionine (SAMe) in the liver. It has been shown that betaine administration has the capacity to elevate hepatic levels of SAMe and to prevent ethanol-induced fatty liver[1]. Kanbak et al[2] reported that betaine protects the liver from ethanol-mediated hepatotoxicity in rats, and it was reported recently that it also protects the liver from ischemia/reperfusion injury, niacin toxicity, CCl4-, hyperosmolarity-, and ethanol-induced apoptosis.
Alcohol consumption is associated with the nutritional metabolism system, which reduces hepatic glucose production and insulin secretion, and increases protein catabolism and triacylglycerol levels. The liver is predominantly responsible for ethanol metabolism and it appears to be the primary source of blood-borne insulin-like growth factor (IGF)-I. Impairment of hepatic synthesis could be responsible for at least a portion of the alcohol-induced decrease in serum levels of IGF-I. Alcohol can affect various organ conditions, thus alcohol consumption may regulate IGF, IGF binding protein (IGFBP) and the IGF-I receptor, which may be involved in growth disorders. It has been shown that IGFBPs are associated with alcohol metabolism[3,4], and we found recently that alcohol administration not only reduces the level of IGF-I, but also increases the level of IGFBP-1 in rat liver, kidney, and serum[5]. It may also contribute to the metabolic dysfunction that occurs following chronic alcohol consumption. Acute alcohol ingestion also reduces the level of IGF-I in serum, but promptly increases serum IGFBP-1 levels in human subjects[3]. In a pilot study, we observed a reduction in the level of IGF-I in alcohol-treated primary rat hepatocytes.
Based on these previous results, we can speculate that betaine will affect IGF systems in the liver. However, the effects of betaine on the ethanol-mediated secretions of IGF-I and IGFBP-1 in primary cultured rat hepatocytes remain poorly understood. In the present study, therefore, we investigated the effects of betaine on the ethanol-mediated secretions of IGF-I and IGFBP-1 in primary rat hepatocytes.
IGF-I antigen, and IGF-I and IGFBP-1 antibodies were purchased form Gropep (Thebarton, Australia). The mitogen-activated protein kinase (MAPK) inhibitor PD98059 was purchased from New England Biolabs (Beverly, MA, USA), and antibodies against phosphor-p42/44 MAPK, p42/44 MAPK were obtained from Cell Signaling (Beverly, MA, USA). All routine culture media were obtained from Gibco-BRL (Grand Island, NY, USA). Aqualsol, reflection X-ray film, and I125 (isotope) were purchased from Dupont-NEN. Immobilon-P polyvinylidene difluoride (PVDF) membranes were purchased from Millipore. Bovine serum albumin (fraction V), glycine, sodium dodecylsulfate (SDS), acrylamide, glycerol, and Tween 20 were obtained from Sigma Co. (St. Louis, MO, USA).
Hepatocytes were isolated from male Sprague-Dawley rats weighing 200-300g by a two-step perfusion procedure with 0.5g/L collagenase that has been described previously (Seglen, 1976: Weng and Shukla, 2000). Cell viability, as assessed by exclusion of trypan blue, was 90% ± 5%. Isolated hepatocytes were plated onto collagen-coated plastic culture dishes (60 mm in diameter) at a density of 5 × 104 cells/cm2 in William’s medium E containing 100ml/L fetal bovine serum (FBS) in an incubator with an atmosphere of 50ml/L CO2. After 3 h, the medium was replaced with FBS-free-Williams medium E and various concentrations of ethanol and PD98059 were carefully added to the cells. The dishes immediately were sealed tightly with parafilm and the cells were incubated in this way for 10-180 min at 37°C.
Cells were rinsed twice with ice-cold phosphate-buffered saline and lysis buffer was added (20 mmol/L HEPES, pH 8.8, 136 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L EGTA, 10 g/L Triton X-100, 10 mmol/L KCl, 2 mmol/L MgCl, 1 mmol/L phenylmethylsulfonyl fluoride, 1 mmol/L sodium orthovanadate, 1 mmol/L dithiothreitol, 1 mmol/L benzamidine, 10-B-glycerophosphate, 10 mg/L aprotinin, 10 mg/L leupeptin, and 1 mg/L pepstatin A). Cell lysates were collected and sonicated for 5 min in a VibraCell ultrasonic processor. After centrifugation of the sonicated samples at 12 000 ×g for 10 min at 4°C, the supernatant was collected and protein concentrations were estimated using a bicinchoninic acid assay kit.
Recombinant human IGF-I was iodinated to a specific radioactivity of 5.55 - 11.10 MBq/g with the isotope I125 using a modified version of the chloramin-T method (Kodak, NY, USA). The specific activity of the iodinated IGF-I was approximately 2.22 - 4.07 MBq/g protein. The iodination mixture was purified on a Sephadex G-50 column (150 cm) and pre-equilibrated with phosphate-buffered saline (0.1 mol/L, pH 7.4). Serum and tissue IGFBPs were separated using the method of Lee et al[6], and IGF-I immunoreactivity was determined using the method of Lee et al[7]. IGF-I data are expressed in terms of nanograms of pure human IGF-I per milliliter assuming equal cross-reactivity of rat and human IGF-I in the RIA. Fifty microliters of rat polyclonal IGF-I antibody diluted to 1:1,500 was added to 100 μL of each sample/standard and then incubated for 1 h at room temperature. [125I]-IGF-I (20000 cpm) was then added, and the sample/standard was incubated for an additional 18 h at 4°C. Fifty microliters of horse serum (Sigma) was added to the incubated sample, which was then centrifuged at 3000g for 30 min. The supernatant was discarded, and the radioactivity of the precipitate containing bound [125I]-IGF-I was counted in a gamma scintillation counter (Wallac, Finland). All assays were performed in duplicate. Intra- and interassay coefficients of variation for IGFs were 8% and 10%, respectively.
Supernatants were concentrated for 5 h using a Centricon processor (Millipore) at 4°C. Equal amounts of concentrated protein and cell lysates (20-30 µg) were separated on 100 g/L SDS-polyacrylamide gel electrophoresis (PAGE) gels. After electrophoresis, proteins were transferred to a PVDF membrane. The membrane was washed with Tris-buffered saline containing Tween 20 (TBS-T; 25 mmol/L Tris, pH 7.4, containing 137 mmol/L NaCl and 10 g/L Tween-20) and then blocked with TBS-T containing 50g/L nonfat dry milk for 2 h at room temperature. Blots were incubated with antibodies against IGFBP-1 overnight at 4°C and then incubated with anti-rabbit horseradish peroxidase. After washing, the specific protein band was visualized using an enhanced chemiluminescence detection system (Pierce Chemical, IL, USA). For MAPK blotting, cell lysates were separated on 100 g/L SDS-PAGE and blotted with p-p42/44 MAPK antibody.
All experiments were repeated at least three times. The data obtained from this investigation were analyzed using ANOVA and Student’s t test and are expressed as mean ± SD values.
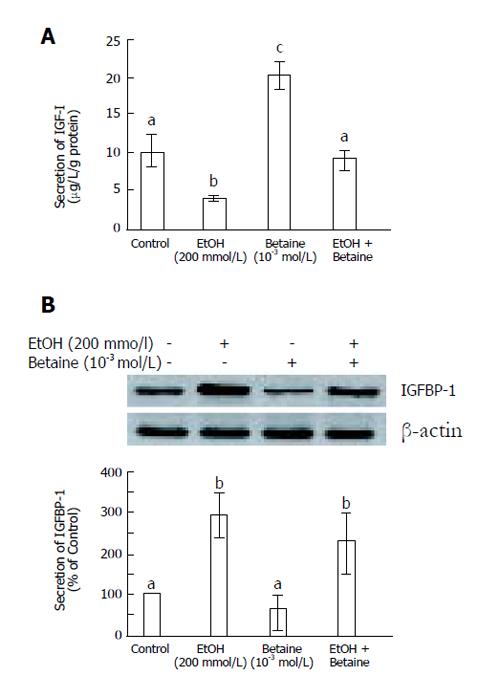
Chronic alcohol treatment is associated with liver and kidney damage. IGF is the major growth factor that is affected by alcohol consumption. Recently, we found that alcohol reduces the level of IGF-I and increased that of IGFBP-1 in the serum, liver, and kidney of Sprague-Dawley rats[5]. To examine the possible effect of betaine, an osmolyte, on the regulation of alcohol-stimulated IGF-I and IGFBP-1 secretion, we measured the secretion of IGF-1 and IGFBP-I after cotreatment with betaine (for 300 min) and alcohol (for 180 min) in primary cultured rat hepatocytes. Whereas the secretion of IGF-I was significantly reduced by alcohol treatment alone, co-treatment with betaine resulted in IGF-I secretion being maintained at control levels (Figure 1A, P < 0.05). In addition, betaine reduced alcohol-stimulated IGFBP-1 secretion (Figure 1B). Taken together, these results suggest that betaine ameliorates the alcohol-mediated secretion of IGF-I and IGFBP-1 in primary cultured rat hepatocytes.
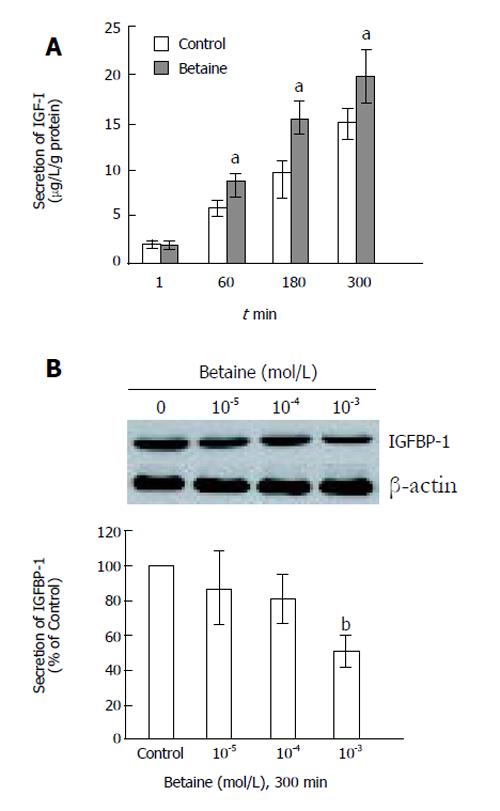
To evaluate the modulatory effects of betaine, we examined its effect on IGF-I and IGFBP-1 secretion in primary rat hepatocytes. Confluent cells were treated with 10-3 mol/L betaine for the times indicated in Figure 2. Supernatant was removed and the levels of IGF-I were measured by RIA (Figure 2A). The secretion of IGF-I increased significantly with increasing time after betaine treatment (Figure 2A, P < 0.05 vs Control). To examine the effect of betaine on IGFBP-1 secretion, rat hepatocytes were treated with the concentrations of betaine indicated in Figure 2B for 300 min, and secretion of IGFBP-1 was monitored. Betaine decreased IGFBP-1 secretion in primary rat hepatocytes in a dose-dependent manner (Figure 2B). In summary, IGF-I secretion was significantly increased by betaine treatment, whereas IGFBP-1 secretion was decreased.
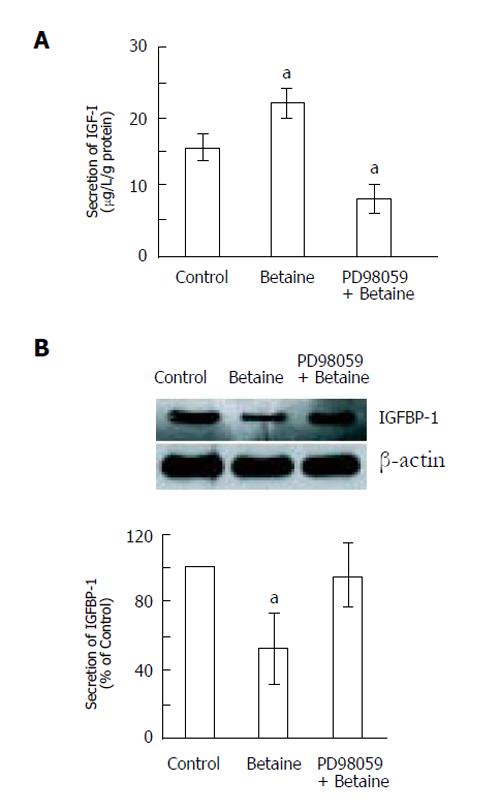
To determine the molecular mechanism by which betaine stimulates IGF-I and reduces IGFBP-1 secretion in rat hepatocytes, we examined the involvement of MAPK. The effects of phosphorylation of p42/44 MAPK on the secretions of IGF-I and IGFBP-1 were monitored for 300 min after treatment with betaine alone (10-3 mol/L) and with betaine (10-3 mol/L) after pretreatment with PD98059 (10 µmol/L). Pretreatment with PD98059 not only significantly decreased betaine-stimulated IGF-I secretion (Figure 3A), but also attenuated the betaine-induced reduction in IGFBP-1 secretion (Figure 3B).
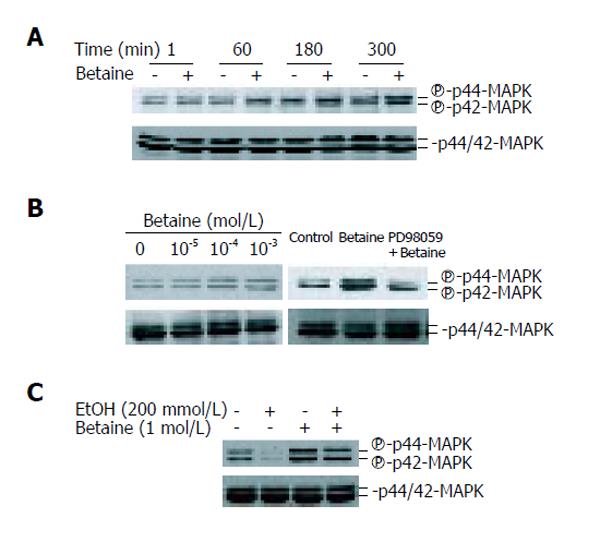
Given the effects of PD98059 on IGF-I and IGFBP-1 secretion, we performed an immunoblot analysis with antibody binding to phosphorylated MAPK 1/2 molecules. Whole lysates of cells treated with betaine were subjected to 100 g/L SDS-PAGE and blotted with phospho-p44/42 MAPK. The phosphorylation of MAPK 1/2 was increased at 60, 180, and 300 min after betaine stimulation, reaching a maximum after 300 min (Figure 4A). Betaine stimulated the phosphorylation of p42/p44 MAPK in a dose-dependent manner (Figure 4B). A significant (P < 0.05) phosphorylation of p42/p44 MAPK was observed at a betaine concentration of 10-3 mol/L compared with the control (Figure 4B). Western blot of anti-phospho-MAPK was then reprobed with a specific antibody for MAPK 1/2. The resulting bands coincided with the immunoreactive bands of MAPK 1/2 (Figure 4B). Betaine ameliorated the activation of MAPK, although alcohol treatment inhibited the basal phosphorylation of p42/44 MAPK (Figure 4C). The effect of betaine on MAPK activation was also monitored at 300 min after treatment of the hepatocytes with betaine (10-3 mol/L); betaine induced the activation of p42/44 MAPK.
Alcohol abuse is associated with deleterious effects on several organs of the body, particularly the liver and brain[8]. Alcohol-induced liver damage is one of the major causes of morbidity and mortality among alcoholics, exposure to ethanol leading to both short- and long-term changes in liver function. Ethanol alters hepatic carbohydrate and lipid metabolism, as well as the synthesis of protein and DNA, which leads to liver dysfunction and cirrhosis[9]. The mechanisms and mediators underlying this liver injury, however, are not clearly understood. Kanbak et al[2] reported that betaine protects the liver from ethanol-mediated hepatotoxicity in rats, and it was reported recently that betaine also protects the liver from ischemia/reperfusion injury, niacin toxicity, and CCl4-, hyperosmolarity-, and ethanol-induced apoptosis.
In the present study we found that the secretion of IGF-I, which is inhibited by ethanol, is increased by treatment with betaine, whereas that of IGFBP-1, which is increased by ethanol is reduced by betaine. It has been reported recently that lipid peroxidase activity is involved in the effects of ethanol on the secretions of IGF-I and IGFBP-1, but the underlying mechanism remains to be elucidated[10]. It has also been reported that acute ingestion of ethanol raises the plasma levels of IGFBP-1 in normal subjects[3,11]. Intake of ethanol influences the bioavailability of IGF-I in normal individuals. Barak et al[1] has shown that betaine, which is a naturally occurring substance, may be used as a protective agent against hepatotoxic substances such as ethanol and CCl4. It may prevent the generation of reactive oxygen species in hepatocytes that results from treatment with ethanol, which is thought to be responsible for attenuation of IGF-I and increase in IGFBP-1 secretion.
This study also aimed to determine whether changes in IGF-I and IGFBP-1 secretion induced by betaine involve signal transduction in hepatocytes. Dose-dependent influences of betaine on the secretion of IGF-I and IGFBP-1, and on MAPK activity were observed. A significant increase in the secretion of IGF-I, a decrease in the secretion of IGFBP-1, and a significant increase in the phosphorylation of p42/p44 MAPK were observed 300 min after treatment with 200 mmol/L ethanol. The activation of p42/44 MAPK was blocked by the MAPK inhibitor PD98059 (10 µmol/L), and this greatly influenced the secretion of IGF-I and IGFBP-1.
In conclusion, the modulation of hepatocyte secretion of IGF-I and IGFBP-1 by betaine involves alteration of p42/44 MAPK activity; betaine may prevent ethanol-induced changes in IGF-I and IGFBP-1 secretion in hepatocytes.
S- Editor Pan BR L- Editor Zhang JZ E- Editor Ma WH
| 1. | Barak AJ, Beckenhauer HC, Tuma DJ. S-adenosylmethionine generation and prevention of alcoholic fatty liver by betaine. Alcohol. 1994;11:501-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Kanbak G, Inal M, Baycu C. Ethanol-induced hepatotoxicity and protective effect of betaine. Cell Biochem Funct. 2001;19:281-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Rojdmark S, Rydvald Y, Aquilonius A, Brismar K. Insulin-like growth factor (IGF)-1 and IGF-binding protein-1 concentrations in serum of normal subjects after alcohol ingestion: evidence for decreased IGF-1 bioavailability. Clin Endocrinol (Oxf). 2000;52:313-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Nedic O, Nikolic JA, Hajdukovic-Dragojlovic L, Todorovic V, Masnikosa R. Alterations of IGF-binding proteins in patients with alcoholic liver cirrhosis. Alcohol. 2000;21:223-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Park SH, Heo JS, Kang CW. Dose-dependent effect of alcohol on insulin-like growth factor systems in male rats. Clin Exp Pharmacol Physiol. 2004;31:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Lee CY, Henricks DM. Comparisons of various acidic treatments of bovine serum on insulin-like growth factor-I immunoreactivity and binding activity. J Endocrinol. 1990;127:139-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Lee MS, Kang CW, Ryu H, Kim JD, Chung HT. Effects of ChunDoSunBup Qi-training on growth hormone, insulin-like growth factor-I, and testosterone in young and elderly subjects. Am J Chin Med. 1999;27:167-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Reddy MA, Shukla SD. Potentiation of mitogen-activated protein kinase by ethanol in embryonic liver cells. Biochem Pharmacol. 1996;51:661-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Lieber CS. Mechanism of ethanol induced hepatic injury. Pharmacol Ther. 1990;46:1-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 123] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Lee SM, Kang CW. Effects of ethanol on secretion of insulin-like growth factor-I (IGF-I) and insulin-like growth factor binding protein-1 (IGBP-1) in primary rat hepatocytes: Involvement of lipid peroxidase (LPO) activity. Korean J Lab Animal Sci. 2004;20:391-397. |
| 11. | Knip M, Ekman AC, Ekman M, Leppaluoto J, Vakkuri O. Ethanol induces a paradoxical simultaneous increase in circulating concentrations of insulin-like growth factor binding protein-1 and insulin. Metabolism. 1995;44:1356-1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |