Published online Mar 21, 2006. doi: 10.3748/wjg.v12.i11.1706
Revised: December 17, 2005
Accepted: December 13, 2005
Published online: March 21, 2006
AIM: To evaluate the long-term histological outcome of patients transplanted for HBV-related liver disease and given HBIg prophylaxis indefinitely after LT.
METHODS: Forty-two consecutive patients transplanted for hepatitis B were prospectively studied. HBsAg, HBV-DNA and liver function tests were evaluated in the serum 3, 6 and 12 mo after LT and then yearly. LB was obtained 6 and 12 mo after LT and yearly thereafter. Chronic hepatitis (CH) B after LT was classified as minimal, mild, moderate or severe.
RESULTS: HBV recurred in 7/42 (16.6 %) patients after 6-96 mo of follow-up. A hundred and eighty-seven LB were evaluated. Four of 7 patients with graft reinfection, all with unknown HBV DNA status before LT, developed cirrhosis at 12-36 mo of follow-up. Of the 122 LB obtained from 28 HBsAg+/HCV- recipients with no HBV recurrence after LT, all biopsies were completely normal in only 2 patients (7.1 %), minimal/non-specific changes were observed in 18 (64.2 %), and at least 1 biopsy showed CH in the remaining 8 (28.5 %). Twenty-nine LB obtained from 7 patients transplanted for HBV-HCV cirrhosis and remaining HBsAg- after LT revealed recurrent CH-C. Actuarial survival was similar in patients with HBsAg+ or HBsAg- liver diseases.
CONCLUSION: Though protocol biopsies may enable the detection of graft dysfunction at an early stage, the risk of progression and the clinical significance of these findings remains to be determined.
- Citation: Targhetta S, Villamil F, Inturri P, Pontisso P, Fagiuoli S, Cillo U, Cecchetto A, Gianni S, Naccarato R, Burra P. Protocol liver biopsies in long-term management of patients transplanted for hepatitis B-related liver disease. World J Gastroenterol 2006; 12(11): 1706-1712
- URL: https://www.wjgnet.com/1007-9327/full/v12/i11/1706.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i11.1706
Hepatitis B virus (HBV)-related liver disease is a common indication for liver transplantation (LT)[1,2] . Before the advent of effective prophylaxis, the rate of HBV recurrence after LT was reportedly more than 90 % at one year in viremic patients and around 30 % in those who were negative for serum HBV-DNA at the time of LT[3]. The majority of patients with recurrent infection develop progressive liver damage resulting in graft and patient survival rates being reduced to around 50 % at 2 years after LT[4,5]. The prevention of HBV recurrence is based on long-term treatment with hepatitis B immunoglobu-lin (HBIg), with or without lamivudine[1,6-9,10-15]. The major drawback of using HBIg is its high cost, while the efficacy of lamivudine is limited by the onset of drug re-sistance[13,14,16-18].
The most reliable way to diagnose and establish the severity of any liver disease recurrence is by histological evaluation of the graft. Serial liver biopsies (LB) may identify subclinical histological changes in LT recipients with normal biochemical data and are useful in assessing disease progression during long-term follow-up[19]. At most centers, liver biopsies are performed in patients with graft dysfunction due to HBV reinfection. There is limited experience of protocol biopsies in patients after LT for HBV. The aim of the present study was to evaluate the usefulness of protocol liver biopsies in assessing long-term histological outcome in patients on indefinite HBIg prophylaxis following LT for hepatitis B.
From November 1990 to December 2000, 246 adults underwent 268 LT at our center, and 55 (22.3 %) of them were hepatitis B surface antigen (HBsAg)-positive. Among the patients with HBV infection, 45 (81.8 %) had cirrhosis and 10 (18.2 %) had fulminant hepatic failure (FHF). Eight patients with cirrhosis also had hepatocellular carcinoma (HCC), 6 were diagnosed pre-LT and 2 were incidental findings. HBV DNA detection methods became available at our center in August 1991. Since then, patients with HBV-related cirrhosis have only been listed for LT if their serum HBV DNA was negative. HBV DNA was unavailable for 5 cirrhotic patients transplanted before August 1991 and for all patients with FHF. Hepatitis delta virus (HDV) antibodies (anti-HDV IgG) were positive in 14 patients (25.4 %), hepatitis C virus (HCV) antibodies in 7 (12.7 %). Three patients (5.4 %) had a history of alcohol abuse but had all abstained for at least 6 mo before LT. Eleven patients who died within 6 mo of LT (5 with cirrhosis and 6 with FHF) and 2 patients with a follow-up of <6 mo (both with cirrhosis) were excluded from the analysis.
Liver tests and serological/virological tests for HBV, HCV and HDV were obtained for all patients 6 and 12 mo after LT and yearly thereafter. Enzyme immunoassays were used to detect HBsAg (ELISA II Abbott Diagnostics, North Chicago, IL), anti-HDV (ELISA II Abbott Diagnostics) and anti-HCV (ELISA II-III, Ortho Diagnostics Raritan, NJ). Samples positive for anti-HCV were confirmed by recombinant immunoblotting assays (RIBA II, Ortho Diagnostics Raritan, NJ). Serum HCV RNA was investigated by RT-PCR and HBV DNA by chemiluminescence (Digene Hybrid Capture System) in serum samples and by PCR in liver tissue using primers from the conserved region of the surface gene of HBV[20].
Liver biopsies were performed using a modified Menghini needle (16-17 gauge). Informed consent for liver bio-psy was obtained from all patients. Hematoxylin and eosin, periodic acid-Schiff (PAS), van Gieson, reticulin and iron stains were available for all biopsy samples. Immunohistological staining was done for HBsAg and HBcAg.
Chronic viral hepatitis was defined as minimal, mild, moderate or severe according to Scheuer and Desmet[21-23]. Recurrent hepatitis C was graded according to Ishak’s classification[24]. Acute and chronic rejection was classified according to Snover’s classification[25]. Minimal and not-otherwise-specified changes were defined according to Pappo’s classification[26].
Patients received 10 000 IU of iv HBIg (VENBIG, Hardis) during the anhepatic phase of LT, daily from days 1 to 7 and weekly for 4 wk following LT. Long-term immunoprophylaxis consisted of weekly doses of 1600 IU of im HBIg (IMMUNOHBS, Hardis) indefinitely. Serum anti-HBs concentrations were investigated weekly before hospital discharge, at 3, 6 and 12 mo after LT and yearly thereafter. Target anti-HBs titers were > 400 IU/L throughout the follow-up. HBIg therapy was discontinued in patients who became HBsAg-positive following LT.
Immunosuppressive therapy was a combination of cy-closporine or tacrolimus with corticosteroids. Azathio-prine or mycophenolate mofetil were added in patients with serum creatinine >200 mol/L. No anti-lymphocyte antibody induction therapy was used. Hist-ologically-proven acute cellular rejection was treated with one or two courses of 1 g/d of iv methylprednisolone for 3 consecutive days.
Lamivudine therapy (100 mg/d) was indicated in patients with detectable serum HBV DNA following LT, with or without reappearance of HBsAg.
Recurrent HBV infection was defined as the detection of HBsAg in the serum at any time after LT.
The Kaplan-Meier method was used to calculate actuarial survivals and the log-rank test for comparisons between groups.
The study population included 42 patients transplanted for hepatitis B with a mean follow-up of 96 mo.
The overall rate of HBV recurrence was 16.6 % (7/42 patients): 18.4 % in patients with cirrhosis (7/38) and 0 % in cases of FHF (0/4) (Table 1). Among the 38 patients with cirrhosis, the HBV recurrence rates varied according to etiology, pre-LT HBV DNA status and presence of HCC. Graft reinfection occurred in 5/19 patients with pure HBV-cirrhosis (26 %), 2/12 patients with HBV/HDV cirrhosis (16.6 %) and none of 7 with HBV/HCV-cirrhosis (Table 1). HBV DNA before LT was unavailable in 4 patients, who all developed recurrent infection - as opposed to only 3/34 (8.8 %) with negative HBV DNA (P = 0.013). Finally, HBV recurred in 2/7 patients (28.5 %) with HCC (1 with unknown pre-LT HBV DNA) and 5/31 (16.1 %) without HCC: among the 4 patients with unknown pre-LT HBV DNA status, 2 died of recurrent HBV 17 and 44 mo after LT, 1 developed graft cirrhosis after 2 years and 1 had moderate chronic hepatitis with a stable clinical course; the remaining 3 patients with HBV recurrence had undetectable HBV DNA prior to LT and became HBsAg-positive 12, 12 and 14 mo after LT. In the first of these 3 patients, HBV recurrence at 12 mo coincided with de novo HCV infection. Although histology revealed mild chronic hepatitis, it was difficult to differentiate the role of HBV and HCV infection in allograft injury. Protocol liver biopsies showed cirrhosis 3, 4 and 5 years after LT. Now, 9 years after LT, without lamivudine treat-ment (which was not indicated because transaminases always remained within the normal range), the patient has compensated cirrhosis.
| Indication | n | HBV DNA | Total recurrence | |
| Negative | Unknown | |||
| Cirrhosis | 38 | 34 | 4 | 7 |
| HBV | 19 | 16 | 3 | 5 |
| HBV-HDV | 12 | 11 | 1 | 2 |
| HBV-HCV | 7 | 7 | ||
| Fulminant hepatitis | 4 | 4 | ||
The second patient, though non-replicating at the time of LT, had intermittently positive HBV-DNA while on the waiting list. At the time of HBV recurrence, 12 mo after LT, liver biopsy showed mild chronic hepatitis. Lamivudine led to serum HBV DNA clearance after 2 mo of treatment. Repeated liver biopsies 2 and 3 years after LT showed stable, mild chronic hepatitis with focal cytoplasmic HBsAg on immunohistochemistry.
The third patient, who had HDV coinfection, developed mild chronic hepatitis 14 mo after LT and was treated with lamivudine.
Six of 7 patients with HBV-HCV coinfection developed histologically-proven recurrent HCV 12 to 48 mo after LT.
Liver HBV DNA, obtained from the first 11 transplanted patients, was positive in 1 patient who had recurrent positive serum HBsAg.
HBIg administration was well tolerated in all patients and no episodes of immunocomplex syndrome were observed. Mean anti-HBs titers 5 years after LT were 713 ± 314 mIU/mL.
Only 2 of the 7 patients with recurrent HBV received lamivudine treatment because it was unavailable in 1991 and 1992 when the other 5 developed graft reinfection. Both patients were cleared of serum HBV-DNA after 6 mo of lamivudine therapy; they are both still taking the treatment with no evidence of any development of the escape mutants.
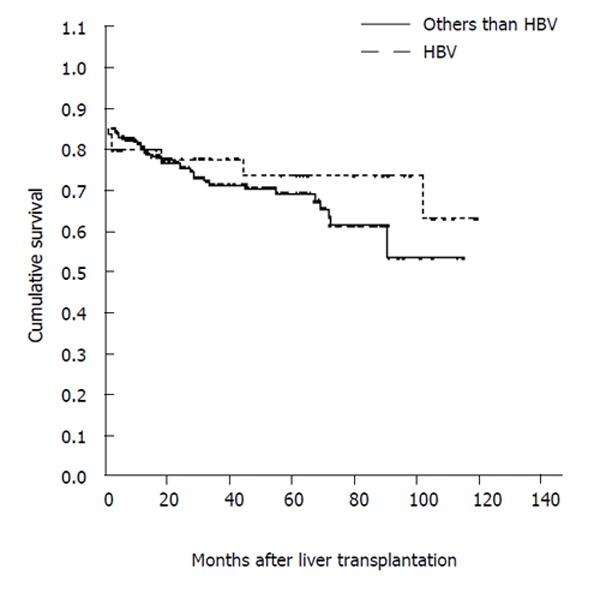
Actuarial survival was similar among patients with HBsAg-positive or HBsAg-negative liver disease (Figure 1), while it was significantly lower (P = 0.009) in patients who had LT for fulminant HBV hepatitis than in those transplanted for HBV cirrhosis, HBV/HDV cirrhosis or HBV/HCV cirrhosis. Moreover, it was not significantly different in patients transplanted for HBV cirrhosis with vs without HCC, despite a decreasing trend in survival 100 mo after LT in patients with HCC (70 % vs 88 %).
The results of the 187 protocol biopsies performed were analyzed depending on the patients’ HBV and HCV status after LT. A hundred and fifty-eight biopsies were obtained from the 35 HCV-negative recipients (mean 4.5 biopsies per patient; range 1-10) during the 6-96 months of follow-up: 36 from 7 patients with recurrent HBV (5.1 per patient; range 2-10) and 122 from 28 patients with no HBV recurrence (4.3 per patient; range 1-11).
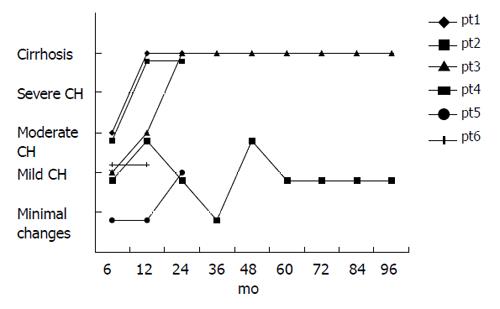
Among the 6 HBsAg+/anti-HCV- patients (there were originally 7, but 1 became anti-HCV+ a year after LT and was consequently included in the HBsAg+/anti-HCV+ group), two had signs of mild chronic hepatitis in all biopsies (follow-up 6-24 mo), and one had at least 1 biopsy showing moderate chronic hepatitis (follow-up 6-96 mo). The other 3 patients, all with HBV-DNA unavailable before LT, developed cirrhosis (two at 12 mo and one at 24) (Figure 2).
As for the 28 HBsAg-/anti-HCV- patients (follow-up for 6-96 mo), biopsies were normal in 2 (7.1%), intermittent, mild inflammatory changes (follow-up for 6-96 mo) were observed in 18 (64.2 %), at least 1 biopsy showed mild chronic hepatitis (follow-up for 6-84 mo) in 6 (21.4 %) and at least 1 biopsy revealed moderate chronic hepatitis (follow-up for 6-84 mo) in 2 (7.1 %). None of the patients in this group had severe chronic hepatitis or cirrhosis. Only 3 patients were HBeAg+ and due to this small number, no biochemical or histological correlations between HBeAg+ and anti-HBeAg+ patients were performed.
The histological features of the 28 patients without HBV recurrence are shown in Table 2. Twenty-nine biopsies in all were obtained from the 7 HCV+/HBsAg- patients during 6-60 mo of follow-up (4.1 biopsies per patient; range 2-6). All 7 patients developed chronic hepatitis with fibrosis 12 to 48 mo after LT (Table 3). The patient with recurrent HBsAg+, who also became anti-HCV positive 1 year after LT, developed mild chronic hepatitis a year after transplantation and cirrhosis 3 years after LT.
| 6 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| mo | yr | yr | yr | yr | yr | yr | yr | yr | |
| n | 28 | 26 | 19 | 16 | 12 | 11 | 8 | 8 | 4 |
| No. of biopsies/total biopsies (%) | |||||||||
| Negative | 5/26 | 9/23 | 4/19 | 6/14 | 6/12 | 3/10 | 4/8 | 4/8 | 1/2 |
| (19.2%) | (39.1%) | (21.0%) | (42.8%) | (50.0%) | (30.0%) | (50.0%) | (50.0%) | (50.0%) | |
| Minimal, not | 19/26 | 8/23 | 11/19 | 6/14 | 5/12 | 6/10 | 2/83/81/2 | ||
| otherwise specified | (73.0%) | (39.7%) | (57.8%) | (42.8%) | (41.6%) | (60.0%) | (25.0%) | (37.5%) | (50.0%) |
| hanges | |||||||||
| Mild chronic hepatitis | 2/26 | 6/23 | 4/19 | 2/14 | - | - | 1/8 | ||
| (7.6%) | (26.0%) | (21.0%) | (14.2%) | - | - | (12.5%) | |||
| Moderate chronic | - | - | - | - | 1/12 | 1/10 | 1/8 | 1/8 | |
| hepatitis | (8.3%) | (10.0%) | (12.5%) | (12.5%) | |||||
| Severe chronic | - | - | - | - | - | - | - | - | - |
| hepatitis | |||||||||
| Cirrhosis | - | - | - | - | - | - | - | - | |
| Pts | Stage | Grade | Months after transplantation |
| 1 | 1 | 4 | 36 |
| 2 | 2 | 4 | 48 |
| 3 | 1 | 4 | 24 |
| 4 | 2 | 10 | 12 |
| 5 | 0 | 2 | 36 |
| 6 | 2 | 5 | 12 |
| 7 | 1 | 2 | 12 |
Immunohistochemistry was performed on 106/187 (56.7 %) liver biopsies. Focal HBcAg positivity was seen in 4/106 (3.7 %) biopsies, obtained from 4 HBsAg negative patients, all of them HBV-DNA negative at the time of the biopsy and offering no clue as to the clinical relevance of this finding. Focal HBsAg positivity was seen in only 1 patient with recurrent HBV.
Acute cellular rejection was histologically confirmed in 11/42 patients (26.1 %), with a total of 14 episodes (0.33 episodes/patient), 1 to 6 mo after LT; 8 patients had one episode of rejection and 3 patients had two. None of the patients developed steroid-resistant or chronic rejection.
HBV-related liver disease is now a common indication for liver transplantation[1,27,28], since graft and patient survival rates are comparable with those of patients transplanted for other conditions[29]. Although perioperative mortality was high in this study, it was unrelated to recurrent HBV infection. Previous studies have shown that the outcome of LT is worse in patients with fulminant HBV hepatitis[30,31] and fulminant non-HBV liver failure[32], as confirmed by our findings. The survival rate was similar, however, between HBsAg positive and negative patients transplanted at our center.
The overall HBV recurrence rate in this series (16.6 %) was low and similar to the rate reported in other studies using long-term HBIg monotherapy[14,33-36]. When the analysis was restricted to the cirrhotic cases with negative pre-transplant HBV-DNA, the recurrence rate was even lower (8.8 %). These good results confirm that patients without HBV replication before LT and given long-term immunoglobulin prophylaxis are at low risk of recurrent infection, as amply reported elsewhere[5,37-41]. Our study also shows that iv infusions of HBIg are not required beyond the perioperative period; putatively protective levels of anti-HBs (>400 IU) can be obtained using the im route, as reported elsewhere[42-45].
In our series, the rate of HBV recurrence was similar in
HBsAg-positive patients with and without HDV coin-fection, unlike the situation observed in previous publi-cations[28,46-49], where HDV coinfection was associated with
a lower rate of HBV recurrence and improved survival.
Only 2 of our patients with HBV/HDV-cirrhosis develop-ed recurrence, however, and one of them had unknown (but probably positive) pretransplant HBV-DNA.
Similarly, although HBV recurred more frequently in cirrhotic patients with HCC, as in previous publications [50-52], the difference was not statistically significant. In addition, one of the 2 HCC patients with HBV recurrence was intermittently positive for HBV-DNA, while the other had unknown (but probably positive) HBV-DNA pre-LT.
The strength of this study lies mainly in the performance of long-term per-protocol serial liver biopsies, the clinical utility of which is still not clear (though the value of protocol biopsies has recently been confirmed[19,53-55]). The International Liver Transplantation Society Expert Panel Consensus Conference on liver transplantation for hepatitis C considered per protocol biopsies essential: all anti-HCV+ liver transplant recipients should undergo annual liver biopsy to determine histological progression and provide additional data on the natural history of the disease[56].
Among the 28 patients treated with HBIg who remained HBsAg and anti-HCV-negative after LT, only 7 % had a completely normal histology up to 3 years after LT. Although the majority of abnormal biopsies showed only minimal inflammatory changes, almost 30 % fulfilled the criteria for the diagnosis of chronic hepatitis, as reported elsewhere[26,55]. The features of mild or moderate chronic hepatitis were seen in nearly 1/3 of our cases.
Protocol biopsies obtained from the 7 HBsAg-negative recipients with post-LT HCV re-infection showed re-current hepatitis C with variable degrees of inflammation and fibrosis. It is worth noting, however, that none of the patients progressed beyond stage 2 fibrosis after a mean follow-up of 36 mo, suggesting that the HBIg preparation used may contain HCV neutralizing antibodies that may attenuate the severity of recurrence, as reported by Feray et al[57], but this is a controversial issue because no other papers have confirmed these data since 1990. HBcAg expression was found in the liver tissue of a minority of biopsies from HBsAg-negative recipients, but it was focal and transient. In addition, the finding of HBV-DNA in liver tissue correlated with serum HBV-DNA positivity. Despite the limited number of cases studied, we feel confident in saying that serum assay is clinically useful, whereas tissue HBV DNA should not be investigated because it is expensive and time-consuming. We did not assess HBV genotypes, but it has been recently reported that genotype C seems to be associated with a higher risk of reactivation of hepatitis B and progression to cirrhosis than genotype B[58]. As previously reported, we observed a low rate of acute rejection among patients transplanted for hepatitis B[59-60]. This may be an indirect consequence, at least in part, of the long-term administration of human polyclonal immunoglobulins, which have immunosuppres-sive properties[59].
In short, this study shows that 25% of patients transplanted for hepatitis B and remaining HBsAg-negative with HBIg prophylaxis develop histological features of mild or moderate chronic hepatitis despite having normal liver test results and negative virological markers[26,55]. These cases will require additional follow-up to estimate the risk of disease progression and further clarify the cause of liver damage. Our findings confirm that normal liver function test results cannot guarantee a healthy graft. The performance of protocol liver biopsies may enable the identification of early histological anomalies potentially capable of progressing to significant liver damage[19,53], but this needs further confirmation.
S- Editor Wang J L- Editor Zhang JZ E- Editor Wu M
| 1. | Vargas HE, Dodson FS, Rakela J. A concise update on the status of liver transplantation for hepatitis B virus: the challenges in 2002. Liver Transpl. 2002;8:2-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Steinmüller T, Seehofer D, Rayes N, Müller AR, Settmacher U, Jonas S, Neuhaus R, Berg T, Hopf U, Neuhaus P. Increasing applicability of liver transplantation for patients with hepatitis B-related liver disease. Hepatology. 2002;35:1528-1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 150] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 3. | Samuel D, Bismuth A, Mathieu D, Arulnaden JL, Reynes M, Benhamou JP, Brechot C, Bismuth H. Passive immunoprophylaxis after liver transplantation in HBsAg-positive patients. Lancet. 1991;337:813-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 274] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 4. | Todo S, Demetris AJ, Van Thiel D, Teperman L, Fung JJ, Starzl TE. Orthotopic liver transplantation for patients with hepatitis B virus-related liver disease. Hepatology. 1991;13:619-626. [PubMed] |
| 5. | Samuel D, Muller R, Alexander G, Fassati L, Ducot B, Benhamou JP, Bismuth H. Liver transplantation in European patients with the hepatitis B surface antigen. N Engl J Med. 1993;329:1842-1847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 746] [Article Influence: 23.3] [Reference Citation Analysis (1)] |
| 6. | Pruett TL, McGory R. Hepatitis B immune globulin: the US experience. Clin Transplant. 2000;14 Suppl 2:7-13. [PubMed] |
| 7. | Krüger M. European hepatitis B immunoglobulin trials: prevention of recurrent hepatitis B after liver transplantation. Clin Transplant. 2000;14 Suppl 2:14-19. [PubMed] |
| 8. | Ilan Y, Nagler A, Zeira E, Adler R, Slavin S, Shouval D. Maintenance of immune memory to the hepatitis B envelope protein following adoptive transfer of immunity in bone marrow transplant recipients. Bone Marrow Transplant. 2000;26:633-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Cirera I, Mas A, Salmeron JM, Jimenez DF, Sanjose A, Navasa M, Rimola A, Roca M, Grande L, Garcia-Valdecasas JC. Reduced doses of hepatitis B immunoglobulin protect against hepatitis B virus infection recurrence after liver transplantation. Transplant Proc. 2001;33:2551-2553. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Markowitz JS, Martin P, Conrad AJ, Markmann JF, Seu P, Yersiz H, Goss JA, Schmidt P, Pakrasi A, Artinian L. Prophylaxis against hepatitis B recurrence following liver transplantation using combination lamivudine and hepatitis B immune globulin. Hepatology. 1998;28:585-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 375] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 11. | Rizzetto M, Marzano A. Posttransplantation prevention and treatment of recurrent hepatitis B. Liver Transpl. 2000;6:S47-S51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Angus PW, McCaughan GW, Gane EJ, Crawford DH, Harley H. Combination low-dose hepatitis B immune globulin and lamivudine therapy provides effective prophylaxis against posttransplantation hepatitis B. Liver Transpl. 2000;6:429-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 186] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 13. | Rosenau J, Bahr MJ, Tillmann HL, Trautwein C, Klempnauer J, Manns MP, Boker KHW. Lamivudine and low-dose hepatitis B immune globulin for prophylaxis of hepatitis B reinfection after liver transplantation possible role of mutations in the YMDD motif prior to transplantation as a risk factor for reinfection. J Hepatol. 2001;34:895-902. [RCA] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 147] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 14. | Marzano A, Salizzoni M, Debernardi-Venon W, Smedile A, Franchello A, Ciancio A, Gentilcore E, Piantino P, Barbui AM, David E. Prevention of hepatitis B virus recurrence after liver transplantation in cirrhotic patients treated with lamivudine and passive immunoprophylaxis. J Hepatol. 2001;34:903-910. [RCA] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 169] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Naoumov NV, Lopes AR, Burra P, Caccamo L, Iemmolo RM, de Man RA, Bassendine M, O'Grady JG, Portmann BC, Anschuetz G. Randomized trial of lamivudine versus hepatitis B immunoglobulin for long-term prophylaxis of hepatitis B recurrence after liver transplantation. J Hepatol. 2001;34:888-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 108] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 16. | Gutfreund KS, Williams M, George R, Bain VG, Ma MM, Yoshida EM, Villeneuve JP, Fischer KP, Tyrrel DL. Genotypic succession of mutations of the hepatitis B virus polymerase associated with lamivudine resistance. J Hepatol. 2000;33:469-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Mutimer D, Pillay D, Shields P, Cane P, Ratcliffe D, Martin B, Buchan S, Boxall L, O'Donnell K, Shaw J. Outcome of lamivudine resistant hepatitis B virus infection in the liver transplant recipient. Gut. 2000;46:107-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 122] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 18. | Perrillo RP, Wright T, Rakela J, Levy G, Schiff E, Gish R, Martin P, Dienstag J, Adams P, Dickson R. A multicenter United States-Canadian trial to assess lamivudine monotherapy before and after liver transplantation for chronic hepatitis B. Hepatology. 2001;33:424-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 310] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 19. | Lucey MR. Serial liver biopsies: a gateway into understanding the long-term health of the liver allograft. J Hepatol. 2001;34:762-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Pontisso P, Morsica G, Ruvoletto MG, Barzon M, Perilongo G, Basso G, Cecchetto G, Chemello L, Alberti A. Latent hepatitis B virus infection in childhood hepatocellular carcinoma. Analysis by polymerase chain reaction. Cancer. 1992;69:2731-2735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Scheuer PJ. Classification of chronic viral hepatitis: a need for reassessment. J Hepatol. 1991;13:372-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1130] [Cited by in RCA: 1196] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 22. | Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer PJ. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994;19:1513-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1582] [Cited by in RCA: 1506] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 23. | Bianchi L, Gudat F, Chronic hepatitis. In: Mac Sween RNM, Anthony PP, Scheuer PJ, Portmann B, Burt AD, eds. Pathology of the Liver; 3rd ed: Edinburgh: Churchill Livingstone 1994; 349-395. |
| 24. | Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3521] [Cited by in RCA: 3780] [Article Influence: 126.0] [Reference Citation Analysis (1)] |
| 25. | Snover DC, Sibley RK, Freese DK, Sharp HL, Bloomer JR, Najarian JS, Ascher NL. Orthotopic liver transplantation: a pathological study of 63 serial liver biopsies from 17 patients with special reference to the diagnostic features and natural history of rejection. Hepatology. 1984;4:1212-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 146] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Pappo O, Ramos H, Starzl TE, Fung JJ, Demetris AJ. Structural integrity and identification of causes of liver allograft dysfunction occurring more than 5 years after transplantation. Am J Surg Pathol. 1995;19:192-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 97] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 27. | Villamil FG. Hepatitis B: progress in the last 15 years. Liver Transpl. 2002;8:S59-S66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Lerut JP, Donataccio M, Ciccarelli O, Roggen F, Jamart J, Laterre PF, Cornu C, Mazza D, Hanique G, Rahier J. Liver transplantation and HBsAg-positive postnecrotic cirrhosis: adequate immunoprophylaxis and delta virus co-infection as the significant determinants of long-term prognosis. J Hepatol. 1999;30:706-714. [RCA] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Hartley P, Petruckevitch A, Reeves B, Rolles K. The National Liver Transplantation audit: an overview of patients presenting for liver transplantation from 1994 to 1998. On behalf of the Steering Group of the UK Liver Transplantation Audit. Br J Surg. 2001;88:52-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Van Hoeck B, De Boer J, Boudjema K, Williams R, Corsmit O, and Terpstra OT on behalf of the EURALT Study Group. Auxiliary versus orthotopic liver transplantation for acute liver failure. J Hepatol. 1999;30:699-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 89] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | de Rave S, Tilanus HW, van der Linden J, de Man RA, van der Berg B, Hop WC, Ijzermans JN, Zondervan PE, Metselaar HJ. The importance of orthotopic liver transplantation in acute hepatic failure. Transpl Int. 2002;15:29-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Fagiuali S, Mirante VG, Pompili M, Gianni S, Leandro G, Rapaccini GL, Gasbarrini A, Naccarato R, Pagliaro L, Rizzetto M. Liver transplantation: the Italian experience. Dig Liver Dis. 2002;34:640-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Terrault NA, Vyas G. Hepatitis B immune globulin preparations and use in liver transplantation. Clin Liver Dis. 2003;7:537-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Huang MA, Lok AS. Natural history of hepatitis B and outcomes after liver transplantation. Clin Liver Dis. 2003;7:521-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Villamil FG. Prophylaxis with anti-HBs immune globulins and nucleoside analogues after liver transplantation for HBV infection. J Hepatol. 2003;39:466-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 36. | Ben-Ari Z, Mor E, Bar-Nathan N, Shaharabani E, Shapira Z, Tur-Kaspa R. Combination hepatitis B immune globulin and lamivudine versus hepatitis B immune globulin monotherapy in preventing recurrent hepatitis B virus infection in liver transplant recipients. Transplant Proc. 2003;35:609-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 37. | Samuel D. Liver transplantation and hepatitis B virus infection: the situation seems to be under control, but the virus is still there. J Hepatol. 2001;34:943-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 38. | Muller R, Samuel D, Fassati LR, Benhamou J-P, Bismuth H, Alexander GJ. ‘EUROHEP’ consensus report on the management of liver transplantation for hepatitis B virus infection. European Concerted Action on Viral Hepatitis. J Hepatol. 1994;21:1140-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 39. | Roche B, Feray C, Gigou M, Roque-Afonso AM, Arulnaden JL, Delvart V, Dussaix E, Guettier C, Bismuth H, Samuel D. HBV DNA persistence 10 years after liver transplantation despite successful anti-HBS passive immunoprophylaxis. Hepatology. 2003;38:86-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 171] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 40. | Doyle HR, Parmanto B, Munro PW, Marino IR, Aldrighetti L, Doria C, McMichael J, Fung JJ. Building clinical classifiers using incomplete observations--a neural network ensemble for hepatoma detection in patients with cirrhosis. Methods Inf Med. 1995;34:253-258. [PubMed] |
| 41. | Terrault N. Management of hepatitis B virus infection in liver transplant recipients: prospects and challenges. Clin Transplant. 2000;14 Suppl 2:39-43. [PubMed] |
| 42. | Yao FY, Osorio RW, Roberts JP, Poordad FF, Briceno MN, Garcia-Kennedy R, Gish RR. Intramuscular hepatitis B immune globulin combined with lamivudine for prophylaxis against hepatitis B recurrence after liver transplantation. Liver Transpl Surg. 1999;5:491-496. [RCA] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 111] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 43. | Mc Gory R. Pharmacoeconomic analysis of HBV liver transplant therapies. Clin Transplant. 2000;14 Suppl 2:29-38. [PubMed] |
| 44. | Barth JT, Pliskin N, Axelrod B, Faust D, Fisher J, Harley JP, Heilbronner R, Larrabee G, Puente A, Ricker J. Introduction to the NAN 2001 Definition of a Clinical Neuropsychologist. NAN Policy and Planning Committee. Arch Clin Neuropsychol. 2003;18:551-555. [PubMed] |
| 45. | Han SH, Martin P, Edelstein M, Hu R, Kunder G, Holt C, Saab S, Durazo F, Goldstein L, Farmer D. Conversion from intravenous to intramuscular hepatitis B immune globulin in combination with lamivudine is safe and cost-effective in patients receiving long-term prophylaxis to prevent hepatitis B recurrence after liver transplantation. Liver Transpl. 2003;9:182-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 59] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 46. | Taniguchi M, Shakil AO, Vargas HE, Laskus T, Demetris AJ, Gayowski T, Dodson SF, Fung JJ, Rakela J. Clinical and virologic outcomes of hepatitis B and C viral coinfection after liver transplantation: effect of viral hepatitis D. Liver Transpl. 2000;6:92-96. [PubMed] |
| 47. | Ottobrelli A, Marzano A, Smedile A, Recchia S, Salizzoni M, Cornu C, Lamy ME, Otte JB, De Hemptinne B, Geubel A. Patterns of hepatitis delta virus reinfection and disease in liver transplantation. Gastroenterology. 1991;101:1649-1655. [PubMed] |
| 48. | Samuel D, Zignego AL, Reynes M, Feray C, Arulnaden JL, David MF, Gigou M, Bismuth A, Mathieu D, Gentilini P. Long-term clinical and virological outcome after liver transplantation for cirrhosis caused by chronic delta hepatitis. Hepatology. 1995;21:333-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 99] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 49. | Tong MJ, Terrault NA, Klintmalm G. Hepatitis B transplantation: special conditions. Semin Liver Dis. 2000;20 Suppl 1:25-28. [PubMed] |
| 50. | Mazzaferro V, Regalia E, Montalto F, Pulvirenti A, Brunetto MR, Bonino F, Lerut J, Gennari L. Risk of HBV reinfection after liver transplantation in HBsAg-positive cirrhosis. Primary hepatocellular carcinoma is not a predictor for HBV recurrence. The European Cooperative Study Group on Liver Cancer and Transplantation. Liver. 1996;16:117-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 51. | Jonas S, Steinmuller T, Tullius SG, Thelen A, Settmacher U, Berg T, Radtke C, Neuhaus P. Increased mortality after liver transplantation for hepatocellular carcinoma in hepatitis B-associated cirrhosis. Transpl Int. 2003;16:33-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 52. | Wong PY, McPeake JR, Portmann B, Tan KC, Naoumov NV, Williams R. Clinical course and survival after liver transplantation for hepatitis B virus infection complicated by hepatocellular carcinoma. Am J Gastroenterol. 1995;90:29-34. [PubMed] |
| 53. | Burra P, Mioni D, Cecchetto A, Cillo U, Zanus G, Fagiuoli S, Naccarato R, Martines D. Histological features after liver transplantation in alcoholic cirrhotics. J Hepatol. 2001;34:716-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 59] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 54. | Maor-Kendler Y, Batts KP, Burgart LJ, Wiesner RH, Krom RA, Rosen CB, Charlton MR. Comparative allograft histology after liver transplantation for cryptogenic cirrhosis, alcohol, hepatitis C, and cholestatic liver diseases. Transplantation. 2000;70:292-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 50] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 55. | Sebagh M, Rifai K, Féray C, Yilmaz F, Falissard B, Roche B, Bismuth H, Samuel D, Reynès M. All liver recipients benefit from the protocol 10-year liver biopsies. Hepatology. 2003;37:1293-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 90] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 56. | Wiesner RH, Sorrell M, Villamil F. Report of the first Inter-national Liver Transplantation Society expert panel consensus conference on liver transplantation and hepatitis C. Liver Transpl. 2003;9:S1-S9. [PubMed] |
| 57. | Feray C, Gigou M, Samuel D, Ducot B, Maisonneuve P, Reynes M, Bismuth A, Bismuth H. Incidence of hepatitis C in patients receiving different preparations of hepatitis B immunoglobulins after liver transplantation. Ann Intern Med. 1998;128:810-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 99] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 58. | Chu CM, Liaw YF. Genotype C hepatitis B virus infection is associated with a higher risk of reactivation of hepatitis B and progression to cirrhosis than genotype B: a longitudinal study of hepatitis B e antigen-positive patients with normal aminotransferase levels at baseline. J Hepatol. 2005;43:411-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 128] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 59. | Farges O, Saliba F, Farhamant H, Samuel D, Bismuth A, Reynes M, Bismuth H. Incidence of rejection and infection after liver transplantation as a function of the primary disease: possible influence of alcohol and polyclonal immunoglobulins. Hepatology. 1996;23:240-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 103] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 60. | Adams DH, Hubscher SG, Neuberger JM, McMaster P, Elias E, Buckels JA. Reduced incidence of rejection in patients undergoing liver transplantation for chronic hepatitis B. Transplant Proc. 1991;23:1436-1437. [PubMed] |