INTRODUCTION
The importance of the intestinal mucosa in the inflammatory and metabolic responses to sepsis, trauma and other critical illnesses is increasingly recognized. Major trauma and shock may initiate a cascade of intestinal events such as intestinal cytokine overproduction[1-3], increased intestinal permeability[4-6], translocation of intestinal bacteria and endotoxins[7]. These events may not only influence the intestinal mucosa itself, but also may affect the function and integrity of remote organs and tissues[8], leading to systemic inflammatory response syndrome (SIRS) and multiple organ dysfunction syndrome (MODS)[9]. Indeed, the gut has been proposed to be the “motor” of MODS in critical illnesses[4,7,10], mainly through the inflammatory response mediated by nuclear factor kappa B (NF-κB) and proinflammatory cytokines[1]. Our previous study demonstrated that traumatic brain injury (TBI) could induce marked damage of intestinal mucosal structure and barrier function[5]. However, the underlying mechanism for the acute gut mucosal injury induced by TBI remains unclear.
NF-κB is a transcription factor that plays a key role in the activation of genes involved in immune and acute phase responses[11,12]. NF-κB can be activated by lesion-induced oxidative stress, bacterial endotoxin or cytokines, and subsequently increases transcriptionally the expression of the genes for many cytokines[13,14], enzymes[15] and adhesion molecules[16], which have been believed to be involved in the acute inflammatory response. Adhesion molecules can recruit inflammatory cells, such as neutrophils, eosinophils, and T lymphocytes, from the circulation to the site of inflammation to release inflammatory mediators responsible for the gut mucosal damage. NF-κB regulates the expression of several genes that encode these adhesion molecules, including intercellular adhesion molecule-1 (ICAM-1) and E-selectin[16-18], which mediates the initial attachment and rolling of neutrophils, and vascular-cell adhesion molecule-1 (VCAM-1)[19], which mediates the recruitment of monocytes and lymphocytes. Several studies have shown that NF-κB and ICAM-1 play an important role in the pathogenesis of chronic inflammatory bowel disease (IBD)[13,14,20,21]. Taken together, the activation of intestinal NF-κB could induce the up-regulation of ICAM-1, leading to the recruitment of inflammatory cells and exacerbating intestinal inflammation. Intestinal inflammation has been viewed as a process in which gut-derived effector immune cells cause the destruction of other mucosal cells[1,20,22].
To date, there has been no study on intestinal NF-κB binding activity and ICAM-1 expression induced by TBI. Insight into these cellular and molecular events occurring in the gut might contribute to the understanding of inflammatory response and potential mechanisms involved in the acute intestinal mucosal injury following TBI. In this regard, the aim of current study was to evaluate the temporal pattern of intestinal NF-κB activation and ICAM-1 expression following TBI.
MATERIALS AND METHODS
Rat models of TBI
Male Wistar rats (220 to 250 g) were purchased from Animal Center of Chinese Academy of Sciences, Shanghai, China. The rats were housed in temperature- and humidity- controlled animal quarters with a 12 h light/dark cycle, room temperature (RT) at 23±1 °C and free access to water. Physiological parameters and eating behaviors were monitored to avoid the confounding variable of different food intakes between the groups on mucosal responses throughout the experiments. All procedures were approved by the Institutional Animal Care Committee, and were in accordance with the guidelines of the National Institutes of Health on the care and use of animals.
The rats were randomly divided into six groups (6 rats in each group) including control group with sham operation and traumatic brain injury groups at hours 3, 12, 24, and 72, and on d 7 respectively. Following intraperitoneal anesthesia with urethane (1000 mg/kg), the animal head was fixed in the stereotactic device. A right parietal bone window of 5 mm in diameter was made under aseptic conditions with dental drill just behind the cranial coronal suture and beside midline. The dura was kept intact. Right parietal brain contusion was adopted using the weight-dropping method described by Feeney et al[23] and severe traumatic brain injury was made by letting a steel rod weighing 40 g with a flat end diameter of 4 mm fall onto a piston resting on the dura from a height of 25 cm. The piston was allowed to compress the brain tissue a maximum of 5 mm. Our preliminary study showed that there was no significant difference between all control rats at various time points from 3 h to 7 d. Therefore, we chose the rats with sham surgery for 72 h as the control group. The control animals were killed for sample collection at 72 h, and TBI group rats were decapitated at their corresponding time points. A 3 cm segment of the midjejunum was taken, flushed with ice-cold saline, opened longitudinally and stored in liquid nitrogen immediately until use.
Nuclear protein extracts and EMSA
NF-κB was examined using electrophoretic mobility shift assay (EMSA). Nuclear extracts of intestinal tissue were prepared by hypotonic lyses followed by high salt extraction[24]. In brief, about 0.1 g of frozen jejunal tissue was homogenized in 0.8 mL ice-cold buffer A, composed of 10 mmol/L HEPES (pH 7.9), 10 mmol/L KCl, 2 mmol/L MgCl2, 0.1 mmol/L EDTA, 1.0 mmol/L dithiothreitol (DTT), and 0.5 mmol/L phenylmethylsulfonyl fluoride (PMSF) (all from Sigma Chemical Co.). The homogenate was incubated on ice for 20 min, and then 50 μL of 10% Nonidet P-40 solution was added (Sigma Chemical Co.); the mixture was vortexed for 30 s and spun by centrifugation for 1 min at 5000 g, 4 °C. The crude nuclear pellet was resuspended in 200 μL of buffer B, containing 20 mmol/L HEPES (pH 7.9), 420 mmol/L NaCl, 1.5 mmol/L MgCl2, 0.1 mmol/L EDTA, 1 mmol/L DTT, 0.5 mmol/L PMSF, 250 mL/L glycerol, and incubated on ice for 30 min with intermittent mixing. The suspension was spun by centrifugation at 12000 g, 4 °C for 15 min. The supernatant containing nuclear proteins was collected and stored at -70 °C for further analysis. Protein concentration was determined using a bicinchoninic acid assay kit with bovine serum albumin as the standard (Pierce Biochemicals, Rockford, IL).
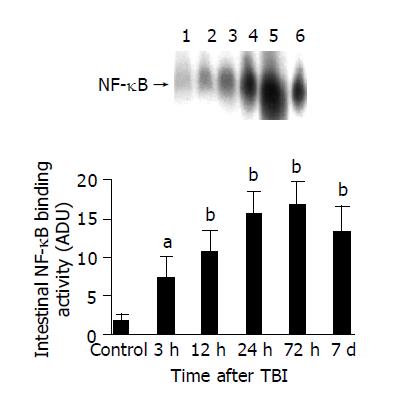
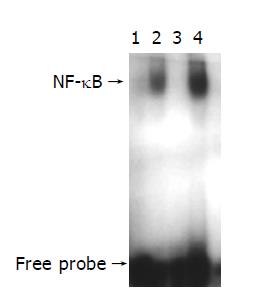
EMSA was performed using a commercial kit (Gel Shift Assay System, Promega, Madison, WI). NF-κB consensus oligonucleotide probe (5’-AGTTGAGGGGACTTTCCCAGGC-3’) was end-labeled with [γ-32P] ATP (Free Biotech, Beijing, China) and T4-polynucleotide kinase. Nuclear protein (20 µg) was pre-incubated in a total volume of 9 µg in a binding buffer, consisting of 10 mmol/L Tris-Cl, pH 7.5, 1 mmol/L MgCl2, 50 mmol/L NaCl, 0.5 mmol/L EDTA, 0.5 mmol/L DTT, 4% glycerol, and 0.05 g/L of polydeoxyinosinic deoxycytidylic acid (dI-dC) for 15 min at room temperature. After addition of the 32P-labeled oligonucleotide probe, the incubation was continued for 20 min at room temperature. Reaction was stopped by addition of 1 µg of gel loading buffer and the mixture was subjected to non-denaturing 4% polyacrylamide gel electrophoresis in a TBE buffer (Tris-borate-EDTA). The gel was vacuum-dried and exposed to x-ray (Fuji Hyperfilm) at -70 °C till an intensifying screen. Levels of NF-κB DNA binding activity was quantified by computer-assisted densitometric scanning and expressed as an arbitrary densitometric unit (ADU).
Detection of ICAM-1 expression in jejunal tissue
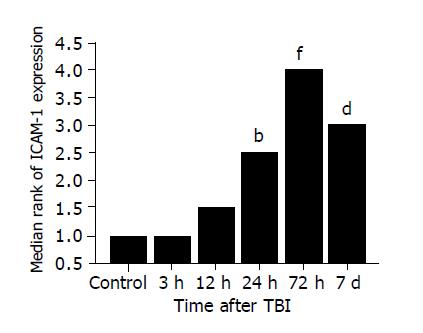
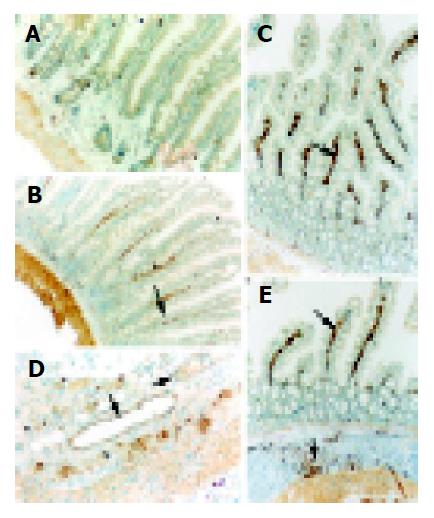
The 10% buffered formalin-fixed jejunal tissue was embedded in paraffin, sectioned in 4 μm thickness with a microtome and stained with hematoxylin and eosin (H-E). The H-E stained sections were examined under a microscope for any alteration in histopathology. Rabbit anti CD54 (ICAM-1) primary antibody was purchased from Boster Biotechnology Co., Ltd, China and the working dilutions for these antibodies were 1:100. For immunohistochemistry, sections were incubated in phosphate-buffered saline (PBS) with 5% normal horse serum and 0.3% Triton X-100 for 1 h at RT. Sections were washed three times with PBS and incubated with primary antibody to ICAM-1 for 2 h at RT. After washing with PBS, sections were incubated with biotinylated second antibodies for 1 h at RT. Sections incubated in the absence of primary antibody were used as negative controls. Microscopy of the immunohistochemically stained tissue sections was performed by an experienced pathologist blinded to the experimental condition. Evaluation of sections was, therefore undertaken by assessing the intensity of staining (5 grades). “0” indicates that there were no detectable positive cells; “1” indicates very low density of positive cells; “2” indicates a moderate density of positive cells; “3” indicates the higher, but not maximal density of positive cells; and “4” indicates the highest density of positive cells.
Statistical analysis
Software SPSS 11.0 was used in the statistical analysis. Each parameter was presented as mean±SD, and compared using one-way ANOVA analysis of variance, followed by Dunnett T3 post hoc test. The level of significance was set at P<0.05.
DISCUSSION
The most important finding of the present study is that TBI might induce a significant up-regulation of NF-κB activity and ICAM-1 immunoreactivity in the intestine. The activation of NF-κB significantly increased by 3 h following TBI, peaked at 72 h and remained elevated on d 7 post-injury. Otherwise, the expression of ICAM-1 was significantly up-regulated in the intestine by 24 h following TBI and peaked at 72 h post-injury. The peak of NF-κB activation and ICAM-1 expression coincided in time with the period during which secondary mucosal injury of the gut was also at its culmination following TBI[5], indicating that intestinal inflammatory response mediated by NF-κB and ICAM-1 might play an important role in the acute gut mucosal damage induced by TBI.
NF-κB is a protein transcription factor that functions to enhance the transcription of a variety of genes, including cytokines, growth factors, intercellular adhesion molecules, immunoreceptors and acute-phase proteins[12-14]. Under normal conditions, NF-κB is complexed in the cytoplasm, where it is inactive, through an interaction of the p65 or c-Rel subunit with inhibitory proteins termed inhibitory kappa B (IκB)[11]. In response to a variety of extracellular stimuli such as proinflammatory cytokines (e.g., IL-1β, TNF-α), lipopolysaccharides (LPS), mitogens, viral proteins, ionizing radiation and certain chemical agents[12], at least two of the IκB proteins (IκBα and IκBβ) are rapidly phosphorylated by the recently identified IκB kinase complex at two conserved N-terminal residues. Phosphorylated IκBα is then rapidly polyubiquitinated, and targeted for degradation through proteasome. Once IκBα is degraded, the nuclear localization sequence of NF-κB is exposed, allowing its translocation to the nucleus and activation of gene transcription.
To the best of our knowledge, till now no study has been found in the literature on the intestinal NF-κB activation after trauma. Information about the intestinal activation and regulation of NF-κB induced by trauma is not well understood. Concerning the importance of NF-κB in the inflammatory response[13,14] and gut in MODS[4,7,10], it is necessary to explore the temporal pattern of intestinal NF-κB activation under the condition of trauma. Although current study has shown that TBI could lead to an immediate and persistent NF-κB activation in the gut, the potential mechanism underlying the initial and subsequent activation of intestinal NF-κB following TBI remains unclear. De Plaen[25] have previously shown that NF-κB was constitutively active at low levels in the small intestine of rats, mainly as p50 homodimers, and could be activated by PAF in vivo within 30 min and was localized in both epithelial cells and lamina propria cells by immunohistochemical studies. Several factors might promote the activation of intestinal NF-κB, including ischemia-reperfusion, cytokines and reactive oxygen species[26] in the gut. Splanchnic hypoperfusion is a common phenomenon in trauma due to sympathetic response and adaptive regulation of blood supply to vital organs. Therefore, it is highly suggested that gut ischemia and subsequent reactive oxygen species might initiate the NF-κB activation, which can be persistently up-regulated by overproduction of intestinal proinflammatory cytokines following TBI.
Under normal physiological condition, there is no NF-κB activation. After trauma and other pathological stimuli, NF-κB is activated and required for maximal transcription of many cytokines and adhesion molecules[13,14], including TNF-α, IL-6 and ICAM-1, which are thought to be important in the generation of acute inflammatory responses[21,27] and chronic inflammatory bowel disease[13,21]. Several animal models have been developed to evaluate the role of NF-κB in the production of inflammatory events[27-29]. Activation of NF-κB correlates with the expression of mRNA for cytokine-induced neutrophil chemoattractant (CINC), a neutrophil chemotactic chemokine, and these events are followed by an influx of neutrophils into the inflamed site[29]. Neurath[27] recently reported an interesting study in which experimental colitis in mice was effectively blocked by the administration of anti-sense oligonucleotides to the Rel A subunit of NF-κB. These findings support the concept that NF-κB plays a pivotal role in the inflammatory response and regulating NF-κB activation can alter inflammatory events.
The intestinal inflammatory response following trauma is characterized by intestinal recruitments of neutrophils and monocytes by releasing proinflammatory cytokines, elastase and superoxide, which can lead to gut mucosal injury[30]. The adhesion to vascular endothelium and infiltration into the gut mucosa of leukocytes are orchestrated by specific adhesion proteins on both endothelial cells and leukocytes. Up-regulation of adhesion molecule is the basis of priming and activation of leukocytes. ICAM-1 is a member of the immunoglobulin superfamily, which is inducible by NF-κB and inflammatory cytokines such as IL-1β and TNF-α. ICAM-1 might be up-regulated to involve in the adhesion and infiltration of leukocytes into the injured site[31,32]. There is a low expression of ICAM-1 in normal intestinal epithelium. In this study, increased ICAM-1 immunoreactivity was observed in intestinal villi and lamina propria following TBI. Increased induction of ICAM-1 immunoreactivity in jejunal vessels might promote leukocyte adhesion to the intestinal vascular endothelia and the infiltration of leukocytes by interactions between ICAM-1 and a group of CD11/CD18 glycoproteins on leukocytes, and further support the local accumulation and activation of immunocompetent cells, leading to the gut mucosal damage.
Because of the central role of NF-κB in the inflammatory response, many researches are presently focused on different methods by which NF-κB activation can be reduced. Recent studies suggested that NF-κB DNA-binding activity in intestinal mucosa during endotoxemia was reduced by induction of the stress response[33], antioxidants[34,35], some anti-inflammatory compounds including glucocorticoids and salicylates[36]. In addition, local or systemic administration of anti-sense oligonucleotides to the NF-κB subunit p65 decreased intestinal inflammation in murine experimental colitis[27]. This may be a brand-new direction for further research on the control of inflammatory response induced by TBI.
In summary, we have shown that TBI could induce a significant up-regulation of intestinal NF-κB activation and ICAM-1 immunoreactivity. The peak of NF-κB activation and ICAM-1 immunoreactivity coincides in time with the period during which secondary mucosal injury of the gut is also at its culmination following TBI. Inflammatory response mediated mainly by NF-κB and ICAM-1 may play an important role in the pathogenesis of acute gut mucosal damage following TBI.