Published online Dec 28, 2005. doi: 10.3748/wjg.v11.i48.7690
Revised: June 10, 2005
Accepted: June 16, 2005
Published online: December 28, 2005
Polycystic liver disease (PLD) is characterized by the presence of multiple bile duct-derived epithelial cysts scattered in the liver parenchyma. PLD can manifest itself in patients with severe autosomal dominant polycystic kidney disease (ADPKD). Isolated autosomal dominant polycystic liver disease (ADPLD) is genetically distinct from PLD associated with ADPKD, although it may have similar pathogenesis and clinical manifestations. Recently, mutations in two causative genes for ADPLD, independently from ADPKD, have been identified. We report here a family (a mother and her daughter) with a severe form of ADPLD not associated with ADPKD produced by a novel missense protein kinase C substrate 80K-H (PRKCSH) mutation (R281W). This mutation causes a severe phenotype, since the two affected subjects manifested signs of portal hypertension. Doppler sonography, computed tomography (CT) and magnetic resonance (MR) imaging are effective in documenting the underlying lesions in a non-invasive way.
- Citation: Peces R, Drenth JP, Morsche RHT, González P, Peces C. Autosomal dominant polycystic liver disease in a family without polycystic kidney disease associated with a novel missense protein kinase C substrate 80K-H mutation. World J Gastroenterol 2005; 11(48): 7690-7693
- URL: https://www.wjgnet.com/1007-9327/full/v11/i48/7690.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i48.7690
Proof of existence of autosomal dominant polycystic liver disease (ADPLD) as a distinct genetic entity, independently from autosomal dominant polycystic kidney disease (ADPKD), stems from three studies in which isolated familial polycystic liver disease (PLD) was shown to be unlinked to the PKD1 or PKD2 loci[1-3]. In 2000, Reynolds et al[4]. identified a locus for ADPLD on chromosome 19p13.2-13.1. In 2003, two independent groups demonstrated that mutations in PRKCSH, encoding the β-subunit of glucosidase II, a N-linked glycan-processing enzyme in the endoplasmic reticulum, cause isolated ADPLD[5,6]. Given its role in the pathogenesis of ADPLD, this protein has been renamed as hepatocystin[5]. Very recently, Davila et al[7]. found that mutations in SEC63 (chromosome 6q21), encoding a component of the protein translocation machinery in the endoplasmic reticulum, also cause ADPLD. Mutations in PRKCSH and SEC63 probably account for less than one-third of ADPLD cases, indicating that there is at least one more locus associated with this disease[7,8].
We have reported here the first Spanish family with a severe form of ADPLD not associated with ADPKD. The proband manifested displacement of abdominal structures by a massively enlarged liver causing portal hypertension and inferior vena cava compression.
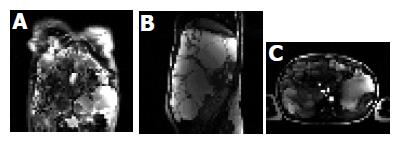
A 36-year-old woman presented with a long history of abdominal enlargement. She had two pregnancies and live births at 29 and 34 years of age, respectively. Physical examination showed a bulging abdomen with a palpable mass extending from the right hemiabdomen to the left quadrant reaching 10 cm below the umbilicus. Laboratory data showed 0.9 mg/dL creatinine (normal range 0.6-1.2 mg/dL), 7.2 g/dL total proteins (normal range 6-8 g/dL), 137 mg/dL total cholesterol (normal range 140-220 mg/dL), 30 mg/dL triglycerides (normal range 50-128 mg/dL), 29 IU/L aspartate aminotransferase (normal range 10-40 IU/L), 22 IU/L alanine aminotransferase (normal range 10-40 IU/L), 135 IU/L gamma glutamyl transpeptidase (normal range 10-40 IU/L), 117 IU/L alkaline phosphatase (normal range 40-105 IU/L), 1.1 mg/dL total bilirubin (normal range 0.1-1.0 mg/dL), 82% prothrombin time and 394 mg/dL fibrinogen (normal range 200-400 mg/dL). Sonography and computed tomography (CT) of the abdomen were compatible with an increased liver volume caused by numerous hepatic cysts of various sizes. Magnetic resonance (MR) imaging of the abdomen revealed a massive polycystic liver with caudal and posterior displacement of the abdominal organs (Figures 1A and B). Doppler sonography and MR angiography demonstrated displacement of the kidneys and their vascular structures by the massively enlarged polycystic liver. The celiac axis was also displaced and the portal vein and a segment of the inferior vena cava below the hepatic veins were compressed by the hepatic cysts (Figure 1C). The kidneys were normal except for two small cortical cysts on the left kidney. There were signs of moderate portal hypertension with varices in the hepatic hilum. Seven years after the diagnosis, the patient was healthy and free of complications. Currently she is awaiting a liver transplantation.
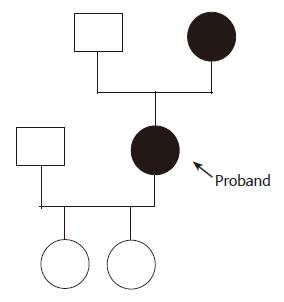
Family history revealed that the patient’s mother also had PLD without kidney disease. The father was not known to suffer from liver or kidney disease. The proband’s daughters had no renal or hepatic cysts. The family pedigree is represented in Figure 2. The proband’s mother (age 72 years) had a history of cholecystectomy in 1983 (performed in another hospital) and a diagnosis of heterozygous beta thalassemia minor in 1990. She had one daughter. Laboratory data showed 37% hematocrit (normal range 36-46%), 12.2 g/dL hemoglobin (normal range 12-16 g/dL), 66.6 fL MCV (80-100 fL), 21.8 pg MCH (normal range 26-34 pg), 5.9% hemoglobin A2 (normal range 2-3.5%), 3 500/μL white blood cell with normal differentiation, 105 000/mm3 platelet count, 0.6 mg/dL creatinine, 6.9 g/dL total proteins, 177 mg/dL total cholesterol, 101 mg/dL triglycerides, 31 IU/L aspartate aminotransferase, 19 IU/L alanine aminotransferase, 14 IU/L gamma glutamyl transpeptidase, 109 IU/L alkaline phosphatase, 2.1 mg/dL total bilirubin, 79% prothrombin time and 362 mg/dL fibrinogen. Ultrasonography and CT of the abdomen demonstrated PLD. There were signs of portal hypertension with varices in the hepatic hilum, gastrohepatic ligament, and lower esophagus. The spleen was enlarged, measuring 17.6 cm in length. The kidneys were normal. The patient was healthy and had no variceal bleeding. She refused any invasive procedure.
DNA was isolated from peripheral blood leukocytes by standard procedures. We performed polymerase chain reaction amplification using specific primer sets for all the 18 exons that constitute the open reading frame of PRKCSH. We determined the nucleotide sequences of the amplified fragments by fluorescence sequencing with dye-terminator chemistry on an ABI3700 capillary sequencer (Perkin-Elmer Applied Biosystems).
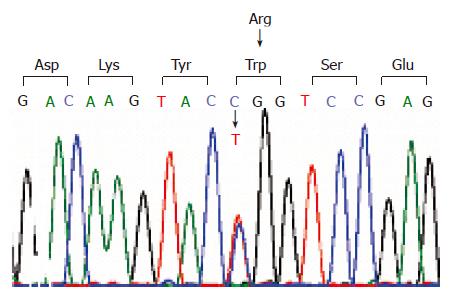
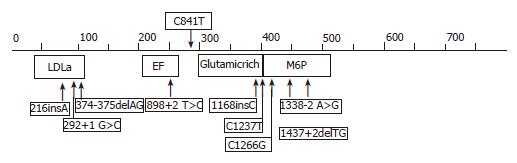
Sequence analysis of PRKCSH gene from DNA isolated from the proband demonstrated a base pair change (C>T) on one allele at position 841 (Figure 3). This mutation located in exon 10 resulted in a change of the amino acid composition of hepatocystin with the replacement of arginine to tryptophan at codon (R281W). The missense mutation was also present in the affected mother of the proband but not in her two children. Within hepatocystin, the mutation was located between the EF-hand calcium-binding domain, ending at codon 234 and the glutamic acid-rich region which starts at codon 299 (Figure 4). Hepatocystin, the protein product of PRKCSH, comprises 528 amino acid residues and has a predicted molecular mass of approximately 59 ku. This protein contains a signal peptide for translocation across the endoplasmic reticulum membrane, a low-density lipoprotein receptor domain class A (LDLa) domain, two EF-hand domains, a glutamic-acid-rich region, a mannose-6-phosphate receptor domain and a conserved C-terminal HDEL amino acid sequence for endoplasmic reticulum retention. Hepatocystin is an endoplasmic reticulum-resident enzyme that is involved in carbohydrate processing and quality control of newly synthesized glycoproteins.
ADPLD is a rare autosomal dominant disorder that has been described in fewer than 50 families from Finnish[8], Dutch[5], American[4], Belgian[2], and Spanish-Belgian[3] ancestry. The pathology of ADPLD consists of numerous cysts of biliary epithelial origin spread throughout the liver parenchyma. This disease is distinct from ADPKD1 and ADPKD2, in which affected individuals develop bilateral renal cysts and liver and pancreatic cysts in advanced cases[9-11]. Cystic liver disease occurs in a substantial portion of patients with ADPKD and end-stage renal disease. Factors influencing the prevalence of PLD in ADPKD include female sex, age (prevalence of PLD is 10% in the third decade, but 60% in patients older than 60 years), and pregnancy (multiparity is associated with increased size and number of cysts in ADPKD)[12].
The recent cloning of the gene involved in ADPLD has greatly facilitated the possibility of a firm molecular diagnosis in our family. Up to now, a total of nine different PRKCSH mutations have been described[13,14]. We detected a novel missense PRKCSH mutation (R281W) in our family. Several lines of evidence suggest that the R281W represents a bona fide disease causing mutation. First, there is segregation of the mutation with the disease in our family. Second, arginine, a positively charged amino acid is replaced by tryptophan which is neutral. Lastly, the C at position 841 is highly conserved among the species (Table 1).
| 278 | 279 | 280 | 281 | |||||||||
| Species | Asp | Lys | Tyr | Arg | ||||||||
| HS | G | A | C | A | A | G | T | A | C | C | G | G |
| BT | G | A | C | A | A | G | T | A | C | C | G | G |
| MM | G | A | C | A | A | G | T | A | C | C | G | C |
| RN | G | A | C | A | A | G | T | A | C | C | G | C |
| Patients | G | A | C | A | A | G | T | A | C | T | G | G |
As is the case for ADPKD, ADPLD is clinically and genetically heterogeneous[8-14]. Although isolated PLD is genetically distinct from PLD associated with ADPKD, both diseases may have similar pathogenesis and clinical manifestations. If ADPLD, like ADPKD[15], occurs by a cellular recessive, two-hit mechanism, then mutations in either PRKCSH or SEC63 will result in loss of proper folding of integral membrane or secreted glycoproteins in bile duct cells that have undergone somatic second hits[7,13,14]. However, the two-hit model has not been investigated in isolated PLD.
The clinical profile of PLD has been defined by Qian et al[16]. who on the basis of a small series concluded that extra hepatic manifestations are infrequent but may include simple renal cysts, intracranial aneurysms, and mitral valve abnormalities. Genotype-phenotype relations remain speculative at this point. Liver cysts are usually asymptomatic, and as a consequence, the disease may go undetected and is likely to be underdiagnosed in the general population[17]. Symptoms occurred are caused by the mass effect of the cysts, or the development of complications such as hemorrhage, infection, or rupture of cysts. Symptoms caused by the mass effect of the cysts include vague abdominal distension, early satiety, dyspnea, and back pain. Rarely, ascites can form because of hepatic venous outflow obstruction by cysts. Lower extremity edema is secondary to extrinsic compression of the inferior vena cava, hepatic veins, or portal vein by large or by many small or medium size hepatic cysts. Compression of the bile ducts can cause jaundice. The symptoms of compression or thrombosis of the inferior vena cava may be obscure and a high index of suspicion is required for the diagnosis[18]. Liver metabolic and synthetic functions remain normal, although an increase in serum levels of alkaline phosphatase, bilirubin and gamma glutamyl transpeptidase, and a decrease in serum levels of total cholesterol and triglycerides have been described in some cases[16]. The factors that affect disease progression are unclear. However, some risk factors for the progression of the disease are age, sex, number of pregnancies, and use of estrogen[14,16].
The proband manifested a massively enlarged liver, which caused abdominal heaviness and progressive hemodynamic changes. She presented displacement of abdominal structures by the massively enlarged liver and hepatic cysts not only caused extrinsic compression of the inferior vena cava, but also were responsible for portal hypertension. Whereas laboratory studies in the proband disclosed a slight increase in the serum levels of alkaline phosphatase and gamma glutamyl transpeptidase, and a decrease in serum levels of total cholesterol and triglycerides, the proband’s mother presented thrombocytopenia and a slight increase in total bilirubin. As the proband’s history shows, the disease may become highly symptomatic in young women. The more severe extensive development of the condition in the proband than in her affected mother contrasts with the reported greater severity of the liver involvement with age. The increased severity of the disease in the proband could be in relation, at least in part, with repeated pregnancies in this patient. However, other possible factors for the progression of the disease remain unknown. Screening of the at-risk members of the families of other affected patients could help to answer this question.
The presence of (few) renal cysts, as demonstrated in our proband who had 2 renal cysts does not preclude the diagnosis ADPLD. This is in accord with the data from another study that demonstrated PRKCSH mutations in four patients with polycystic livers who possessed at least one renal cyst[14].
Most patients with PLD require no treatment. In highly symptomatic patients, percutaneous cyst aspiration, sclerosis and cyst fenestration may be indicated[16]. In patients with diffuse cystic liver, hepatic resection[19] is often the only possibility but entails a high mortality rate. Stent placement in the inferior vena cava has been successfully applied in a few well-selected patients with only inferior vena cava compression. In patients with severe phenotype, liver transplantation should be considered[20]. In our patients, due to the size of their liver, resection of the lobes of their livers for decompression was deemed to be problematic and liver transplantation was considered as the better option in the proband.
In summary, we have described here a family with a severe form of ADPLD not associated with ADPKD, and a novel PRKCSH mutation that was vertically transmitted. The two affected subjects manifested signs of portal hypertension. This family underlines the need for a careful investigation of patients with otherwise unexplained liver cystic disease, focusing on whether other organ systems are involved. It also stresses the importance of accurate family investigation whenever possible. Doppler sonography, CT, and MR are effective in documenting the underlying lesions non-invasively.
We thank the family members for their great cooperation.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Somlo S, Torres VE, Reynolds D, King BF, Nagorney DM. Autosomal dominant polycystic liver disease without polycystic kidney disease is not linked to either the PKD1 or PKD2 gene loci [abstract]. J Am Soc Nephrol. 1995;6:727A. |
| 2. | Pirson Y, Lannoy N, Peters D, Geubel A, Gigot JF, Breuning M, Verellen-Dumoulin C. Isolated polycystic liver disease as a distinct genetic disease, unlinked to polycystic kidney disease 1 and polycystic kidney disease 2. Hepatology. 1996;23:249-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 45] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Iglesias DM, Palmitano JA, Arrizurieta E, Kornblihtt AR, Herrera M, Bernath V, Martin RS. Isolated polycystic liver disease not linked to polycystic kidney disease 1 and 2. Dig Dis Sci. 1999;44:385-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Reynolds DM, Falk CT, Li A, King BF, Kamath PS, Huston J, Shub C, Iglesias DM, Martin RS, Pirson Y. Identification of a locus for autosomal dominant polycystic liver disease, on chromosome 19p13.2-13.1. Am J Hum Genet. 2000;67:1598-1604. [PubMed] |
| 5. | Drenth JP, te Morsche RH, Smink R, Bonifacino JS, Jansen JB. Germline mutations in PRKCSH are associated with autosomal dominant polycystic liver disease. Nat Genet. 2003;33:345-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 161] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 6. | Li A, Davila S, Furu L, Qian Q, Tian X, Kamath PS, King BF, Torres VE, Somlo S. Mutations in PRKCSH cause isolated autosomal dominant polycystic liver disease. Am J Hum Genet. 2003;72:691-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 141] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Davila S, Furu L, Gharavi AG, Tian X, Onoe T, Qian Q, Li A, Cai Y, Kamath PS, King BF. Mutations in SEC63 cause autosomal dominant polycystic liver disease. Nat Genet. 2004;36:575-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 189] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 8. | Tahvanainen P, Tahvanainen E, Reijonen H, Halme L, Kääriäinen H, Höckerstedt K. Polycystic liver disease is genetically heterogeneous: clinical and linkage studies in eight Finnish families. J Hepatol. 2003;38:39-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Coto E, Sanz de Castro S, Aguado S, Alvarez J, Arias M, Menendez MJ, Lopez-Larrea C. DNA microsatellite analysis of families with autosomal dominant polycystic kidney disease types 1 and 2: evaluation of clinical heterogeneity between both forms of the disease. J Med Genet. 1995;32:442-445 DOI : 10.1136/jmg.32.6.442. |
| 10. | Ariza M, Alvarez V, Marín R, Aguado S, López-Larrea C, Alvarez J, Menéndez MJ, Coto E. A family with a milder form of adult dominant polycystic kidney disease not linked to the PKD1 (16p) or PKD2 (4q) genes. J Med Genet. 1997;34:587-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Torres VE, Harris PC. Autosomal dominant polycystic kidney disease. Nefrologia. 2003;23 Suppl 1:14-22. [PubMed] |
| 12. | Everson GT, Taylor MR, Doctor RB. Polycystic disease of the liver. Hepatology. 2004;40:774-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 104] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Drenth JP, Martina JA, Te Morsche RH, Jansen JB, Bonifacino JS. Molecular characterization of hepatocystin, the protein that is defective in autosomal dominant polycystic liver disease. Gastroenterology. 2004;126:1819-1827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Drenth JP, Tahvanainen E, te Morsche RH, Tahvanainen P, Kääriäinen H, Höckerstedt K, van de Kamp JM, Breuning MH, Jansen JB. Abnormal hepatocystin caused by truncating PRKCSH mutations leads to autosomal dominant polycystic liver disease. Hepatology. 2004;39:924-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Watnick TJ, Torres VE, Gandolph MA, Qian F, Onuchic LF, Klinger KW, Landes G, Germino GG. Somatic mutation in individual liver cysts supports a two-hit model of cystogenesis in autosomal dominant polycystic kidney disease. Mol Cell. 1998;2:247-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 134] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Qian Q, Li A, King BF, Kamath PS, Lager DJ, Huston J, Shub C, Davila S, Somlo S, Torres VE. Clinical profile of autosomal dominant polycystic liver disease. Hepatology. 2003;37:164-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 131] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Peces R, González P, Venegas JL. [Polycystic liver disease without autosomal dominant polycystic kidney disease]. Nefrologia. 2003;23:454-458. [PubMed] |
| 18. | Peces R, Gil F, Costero O, Pobes A. [Massive inferior vena cava thrombosis in a patient with autosomal dominant polycystic hepatorenal disease]. Nefrologia. 2002;22:75-78. [PubMed] |
| 19. | Yang GS, Li QG, Lu JH, Yang N, Zhang HB, Zhou XP. Combined hepatic resection with fenestration for highly symptomatic polycystic liver disease: A report on seven patients. World J Gastroenterol. 2004;10:2598-2601. [PubMed] |
| 20. | Gustafsson BI, Friman S, Mjornstedt L, Olausson M, Backman L. Liver transplantation for polycystic liver disease--indications and outcome. Transplant Proc. 2003;35:813-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |