INTRODUCTION
Transmissible spongiform encephalopathies (TSE) are fatal neurodegenerative diseases affecting both animals and human beings[1]. They are characterized by typical cerebral histopathological findings such as amyloid deposition, neuronal loss, and spongiform changes. The prion protein (PrP) can exist in the normal cellular form (PrPc) or in an “infectious” form (PrPsc) that causes disease by converting apathogenic PrPc into pathogenic PrPsc[2]. Previous studies have demonstrated that PrPc is required for prion infection propagation and infectivity has been suggested to be a consequence of conformational modification of PrPc by the infectious PrPsc. Experiment on animals shows that animals lacking the PrPc gene are not able to propagate prion infectivity and are not able to develop the disease[3]. Both the prion isoforms differ dramatically in their physicochemical properties. Whereas PrPc is soluble and easily digested by proteinase K, PrPsc is rich in β-sheet structure, aggregates into fibrils, and is resistant to proteinase K.
The main entry for prions is the gastrointestinal tract. Recent animal studies have shown that after oral exposure to pathogenic prions, PrPsc accumulates in gut lymphoid tissues or in the enteric nervous system prior to its appearance in the central nervous system[4,5]. It has been postulated that prions then propagate from the enteric nervous system along the nerve pathways to ventral and dorsal root ganglia and further through the spinal cord into the brain cortex[4-6].
However, the mechanisms of propagation of prions from the gut lumen, before they reach intestinal lymph follicles or the enteric nervous system remain unexplained. Recently, the 67-ku laminin, binding protein, which can act as a PrPsc receptor was demonstrated on small intestinal epithelial cells[7-9], suggesting that individuals with a high expression of this receptor could be at greater risk of developing TSE after oral challenge with PrPsc. There is also evidence of transepithelial transport of pathogenic prions via intestinal M cells to adjacent lymph follicles (Peyer’s patches)[10]. However, it is unknown if and how far gastrointestinal inflammation may influence PrPc expression and thus potentially PrPsc propagation in the GI tract.
MATERIALS AND METHODS
Patients
Ten H pylori positive patients with non-ulcer dyspepsia (mean age 47 years, range 20-79 years) were included in this study. All patients underwent upper gastrointestinal endoscopy during which four biopsies from the antrum and corpus were obtained. Patients with H pylori gastritis were graded according to the updated Sydney classification[11]. All patients underwent a second endoscopy 4 wk after completing successful eradication therapy consisting of clarithromycine 500 mg twice daily, amoxicillin 1 000 mg twice daily and omeprazole 40 mg twice daily for 1 wk. H pylori was considered to be successfully eradicated, if histology was normal and silver stainable organisms were not detected anymore in the follow-up endoscopy, during which again four biopsies from the antrum and corpus were obtained.
Cell culture
MKN45 and KATO III cell lines were obtained from the American Type Culture Collection. Cells were cultured in RPMI medium containing 10% of fetal calf serum (FCS), 2 mmol/L L-glutamine and antibiotics (1% penicillin-streptomycin, 0.5% gentamycin) at 37 °C in a water-saturated atmosphere of 95% air and 50 mL/L CO2. Subconfluent MKN45 cells were incubated in RPMI without FCS and antibiotics for 24 h. Following serum starvation, cells were exposed to the increasing amounts of gastrin (Clinalfa, Switzerland, C-210), prostaglandin E2 (PGE2), interleukin 1 beta (IL-1β) or tumor necrosis factor alpha (TNF-α) (all from Calbiochem, Bad Soden, Germany).
Extraction of mRNA and RT-PCR analysis
Total RNA was extracted from biopsy specimens and cultured cells using TRIzol reagent (Gibco, Karlsruhe, Germany). Single stranded cDNA was generated from 5 µg RNA using Moloney murine leukemia virus reverse transcriptase (MMLV-RT) and oligo-(dT)-primers (both Stratagene, Heidelberg, Germany). Briefly, 5 µg of total RNA was uncoiled by heating (65 °C for 5 min) and then reverse transcribed (37 °C for 1 h) into complementary DNA (cDNA) in a 50 µL reaction mixture that contained 50 U MMLV-RT, 0.3 µg oligo-(dT)-primer, 40 U RNase Block Ribonuclease Inhibitor, 2 µL of a 100 mmol/L mixture of dNTPs, and 5 µL of buffer (10 mmol/L Tris-HCl, 50 mmol/L KCl, 5 mmol/L MgCl2, pH 8.3). The resultant cDNA (2 µL) was amplified in a 50 µL reaction volume containing 2 U Taq polymerase, dNTP (200 µmol/L each), 1.5 mmol/L MgCl2, 5 µL 10× PCR buffer (50 mmol/L KCl, 10 mmol/L Tris-HCl, pH 8.3) and specific primers at a final concentration of 1 mmol/L (all reagents from Takara, Shiga, Japan). Reactions were carried out at the following conditions: denaturation at 94 °C for 45 s, annealing at 60 °C (for GAPDH) and 67 °C (for PrPc) for 45 s and extension at 72 °C for 2 min. Polymerase chain reaction (PCR) products were detected by electrophoresis on a 1.5% agarose gel containing ethidium bromide. Product size was confirmed by using a 100-bp ladder (Takara, Shiga, Japan) as standard. The gel was photographed under UV transillumination and the intensity of PCR products were measured using a video image analysis system (Kodak Digital Science). The signal for PrPc mRNAs was standardized against that of the GAPDH mRNA from each sample and the results were expressed as PrPc/GAPDH mRNA ratio. The following PCR primers were used based on published sequences: PrPc (sense) 5`- GGCAGTG ACTATGAGGACCGTTAC-3´; PrPc (antisense) 5´-GGCTTGACCAGCATCTCAGGTCTA-3´; GAPDH (sense) 5´-GTCTTCACCACCATGGAGAAGGCT-3´; GAPDH (antisense) 5´-CATGCCAGTGAGCTTCCCGTTCA-3´. Expected product lengths were 528 bp for PrPc and 392 bp for GAPDH. All primer sequences were based on the sequences of the published cDNAs[12,13] and synthesized by GIBCO BRL/Life Technologies (Eggenstein, Germany).
Real-time RT-PCR
PrPc transcript levels were quantified by real-time RT-PCR. Using the Primer Express software (Perkin Elmer, Tokyo, Japan) TaqMan probe and primer set were designed based on published sequences of human PrPc (GenBank accession no.: GI 11079225); PrPc sense (5´-CGCGAGCTTCTCCTCTCCTC-3´), PrPc antisense (5´-GCCCAGGTCACTCCATGT-3´) and beta-2-microglobulin (β2M, GenBank accession no.: XM_007650), β2M sense 5´-TGACTTTGT-CACAGCCCAAGATA-3´, β2M antisense primer 5´-AATCCA-AATGCGGCATCTTC-3´. Probes for PrPc (5´-TCGCCATAA-TGACTGCTCTGCCTCGGT-3´) and β2M (5´-TGATGCTG-CTTACAT GTCTCGATCCCA-3´) were synthesized and labeled with a reporter dye (FAM) at the 5' end and quencher dye (TAMRA) at the 3´-end at MWG Biotech AG (Ebersberg, Germany). For normalization of differences in RNA amounts and efficiencies in the reverse transcription reactions, the housekeeping gene β2M was amplified under the same conditions as PrPc. Real-time RT-PCR was performed on a LightCycler (Roche, Mannheim, Germany) in a reaction volume of 15 µL using the LightCycler FastStart DNA Master Hybridization Probes Kit (Roche Molecular Biochemicals, Mannheim, Germany). The reaction mix included FastStart Taq DNA-Polymerase, dNTP-mix, reaction buffer, MgCl2 (3.0 mmol/L), primers (2 µmol/L of each) and probe (0.5 µmol/L). After pippeting 13.5 µL of this mixture into LC-capillaries and 1.5 µL template cDNA was added. The capillaries were sealed and placed into the thermal chamber of the LightCycler. Samples were amplified with a pre-cycling step at 95 °C for 10 min, followed by 40 cycles of denaturation at 95 °C for 10 s, annealing at 60 °C for 15 s and extension at 72 °C for 6 s.
Immunoblot
MKN45 cells were incubated with Gastrin 1-1 000 nmol/L, PGE2 1-100 nmol/L, TNF-α 1-10 ng/mL or IL-1β 1-10 ng/mL) for 24 h. Cells were collected, washed twice with PBS, and lysed in 0.4 mL of lysis buffer (0.06 mol/L Tris-HCl, pH 6.8, 10% glycerol, 2% SDS, 5% beta-mercaptoethanol, 0.0025% bromophenol blue). DNA was sheared by a needle, the solution heated at 95 °C for 5 min and centrifuged at 15 000 g for 2 min at 4 °C. Twenty-five micrograms of the total protein was loaded on SDS-polyacrylamide gel, run at 40 mA and transferred to nitrocellulose (Protran, Schleicher&Schuell, Germany) by electroblotting. Filters were blocked with 3% bovine serum albumin (BSA, Sigma Aldrich, Germany) in TBS/Tween-20 buffer (137 mmol NaCl, 20 mmol Tris-HCl, pH 7.4, 0.1% Tween-20) before incubation with antibodies against PrPc (mouse monoclonal anti-PrP antibody 6H4, 1:2 000 dilution; Prionics, Switzerland), or β-actin (mouse monoclonal, dilution 1:5 000; Sigma Aldrich, Germany), followed by horseradish peroxidase-conjugated anti-mouse or anti-rabbit-IgG secondary antibody (dilution 1:20.000; Promega, WI, USA) dissolved in 1% non-fat milk in TBS/Tween-20. Immune complexes were detected by the SuperSignal West Pico Chemiluminescent Kit (Pierce, USA) and exposed to an X-ray film (Kodak, Wiesbaden, Germany).
Statistical analysis
Statistical analysis was performed using the Mann-Whitney Wilcoxon's test. The level of significance was set at P<0.05.
RESULTS
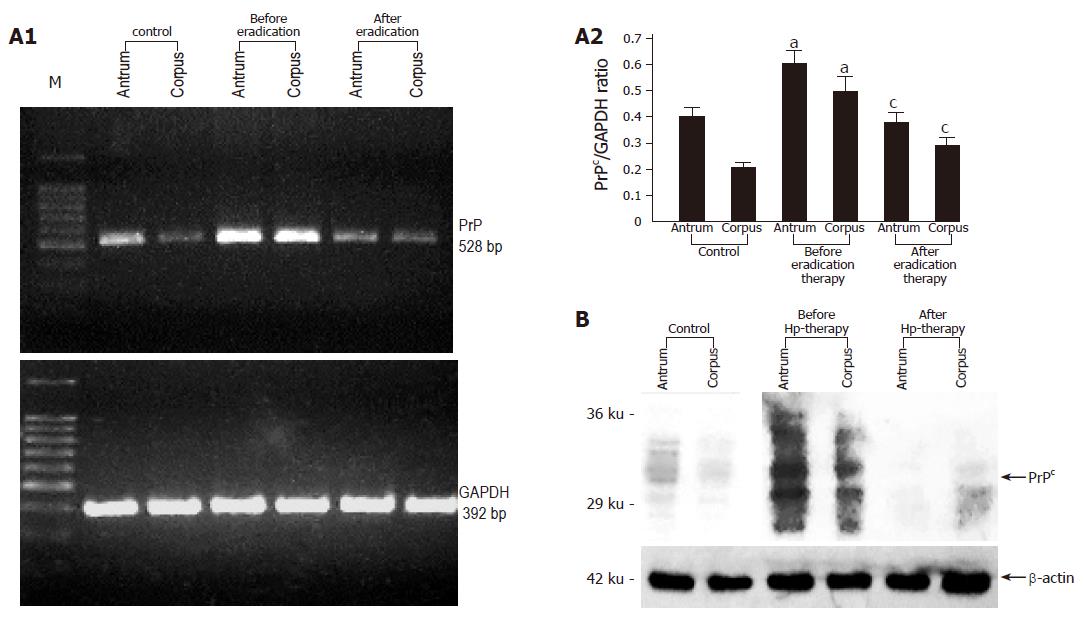
Analysis of gastric biopsy samples obtained endoscopically from patients infected with H pylori, which is found in approximately 50% of the world’s population[14] and which causes gastric inflammation and ulceration[15] demonstrated highly increased PrPc expression compared to uninfected controls. After treatment with antibiotics which usually lead to H pylori eradication, PrPc mRNA and protein expression decreased to control levels (Figures 1A and B).
Figure 1 A: Representative RT-PCR and densitometric analysis showing PrPc mRNA expression in the gastric mucosa colonized with H pylori before and after successful eradication therapy (n = 10).
Data are expressed as means±SE. aP<0.05 vs the control group, cP<0.05 vs the expression before eradication therapy; B: Representative immunoblot analysis showing PrPc protein expression in the gastric mucosa colonized with H pylori before and after successful eradication therapy.
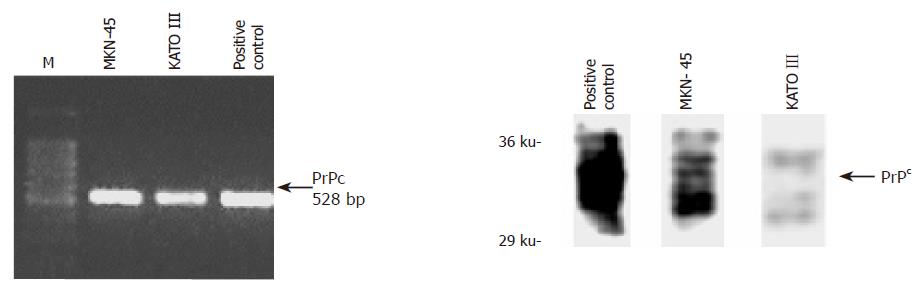
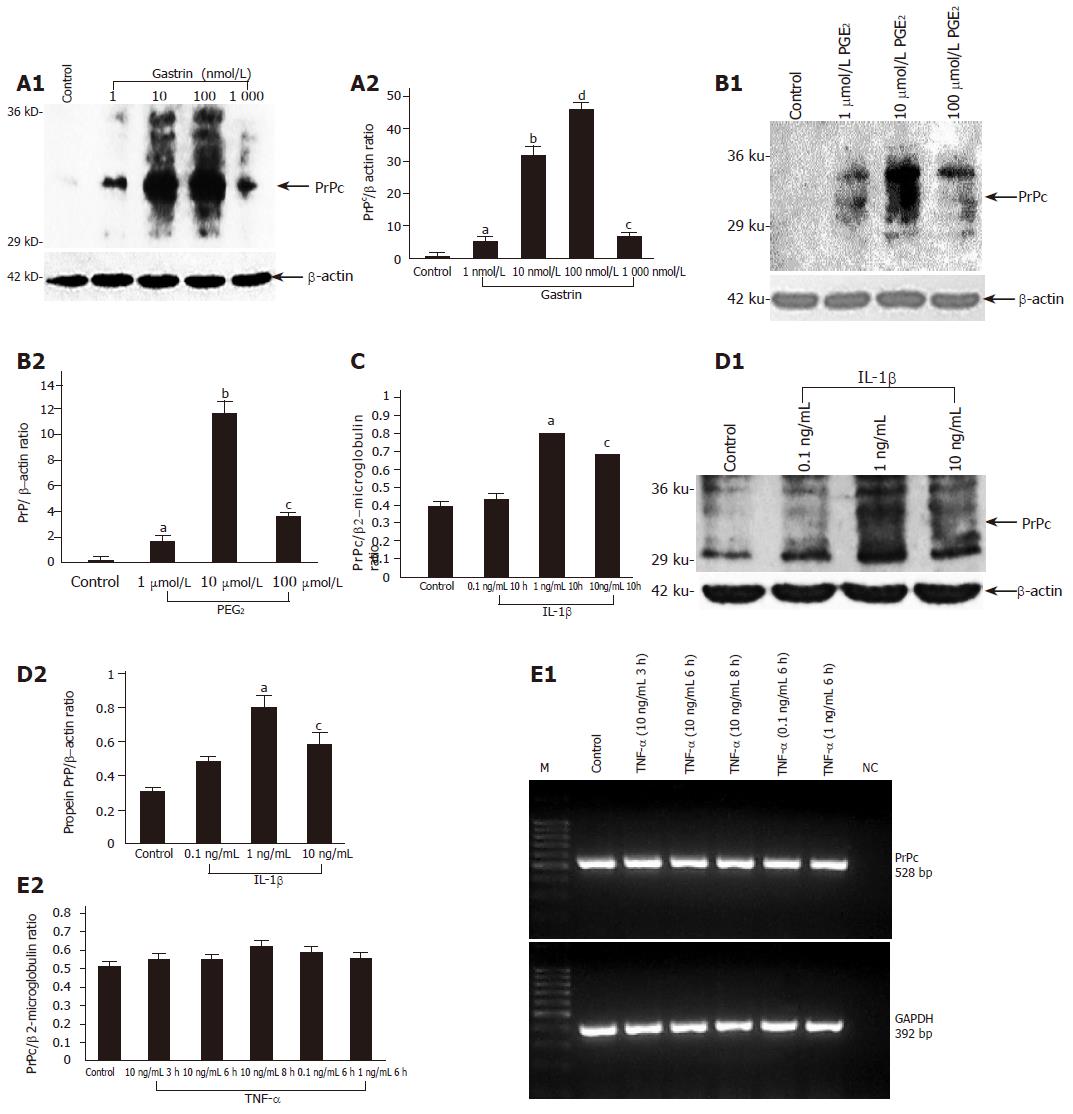
Using RT-PCR and immunoblotting we demonstrated the expression of PrPc mRNA and protein in two different gastric cell lines (MKN45 and KATO III) (Figure 2). In order to assess possible mechanisms responsible for the upregulation of PrPc during chronic H pylori gastritis, we analyzed the effect of increasing doses of key physiological modulators of the gastric mucosa on PrPc expression in these gastric cell lines. These modulators included gastrin (hypergastrinemia is a hallmark of chronic H pylori infection[16,17], PGE2 chronic H pylori infection is accompanied by an increased mucosal production of PGE2[18], and the pro-inflammatory cytokines TNF-α and IL-1β, which are implicated as key promoters of H pylori-induced gastritis and ulceration. When exposed to gastrin, cellular PrPc expression increased in a dose-dependent manner, reaching a peak value of 100 nmol/L (Figure 3A). Similarly, PGE2 induced maximal PrPc mRNA and protein expression at 10 µmol/L (Figure 3B). While IL-1β increased PrPc expression in a dose-dependent manner at the mRNA and protein level (Figures 3C and D), TNF-α showed no effect (Figure 3E).
Figure 2 Protein expression of PrPc in two gastric cell lines (MKN45 and KATO III); the positive control is from bovine brain.
Figure 3 PrPc protein and mRNA expression in MKN45 cells incubated with increasing doses of gastrin (1-1 000 nmol/L) (A), PGE2 (1-100 µmol/L) aP<0.
05 vs control, bP<0.001 vs control, cP<0.05 vs control, dP<0.001 vs control (B), interleukin 1β (0.1-10 ng/mL) (C and D) or TNF-α (0.1-10 ng/mL) aP<0.05 vs control, bP<0.001 vs control, cP<0.05 vs control (E). Data represent means±SE of three independent experiments. At the mRNA level, the expression of PrPc was normalized to β2-microglobulin and at the protein level to β-actin.
DISCUSSION
Considering the importance of the human gastrointestinal tract for the propagation of prions from the gut to the central nervous system, we analyzed the expression of PrPc in human beings with H pylori infection (determined by histology) before and after eradication therapy. This study provides the first in vitro and in vivo evidence that H pylori infection is accompanied by a dramatic upregulation of PrPc expression in the gastric mucosa. The physiological importance of the observed PrPc overexpression in H pylori infected gastric mucosa remains elusive. However, multiple physiological functions of PrPc were identified only recently. Thus, PrPc is involved in signal transduction, from the extracellular space to cells, participates in intracellular signaling and regulation of cell survival, protects against oxidative stress, and interacts preferentially with some of the heat-shock-proteins[19-20]. Therefore, the main role of increased PrPc expression in the stomach appears to be the protection of the gastric mucosa against oxidative stress induced, for example by chronic H pylori infection[21]. This assumption is further supported by a previous study demonstrating that PrPc upregulates antioxidant enzyme activities[22]. Another possible role of PrPc in the gastric mucosa could be the modulation of apoptosis induced by H pylori[23], since PrPc was shown to protect cells against Bax-mediated cell death[24,25]. But this issue remains controversial, as other investigators demonstrated that PrPc may sensitize cells to apoptosis[26,27]. An explanation could be the preferential use of various neuronal cell lines[28] in vitro which necessitates further in vitro and in vivo studies on modulation of apoptosis gastric epithelial cells by PrPc.
In the present study, using quantitative RT-PCR and Western blot analysis we demonstrated an increased PrPc expression in the H pylori infected mucosa which significantly decreased after a successful eradication therapy. The mechanisms behind this phenomenon appears to be linked to the hypergastrinemia observed during H pylori infection which is supported by our in vitro data showing a dose-dependent increase in PrPc expression in gastric epithelial MKN45 cells after incubation with gastrin. According to previous studies, this finding shows that PrPc expression may be modulated by different growth factors[29,30].
Since H pylori infection is associated with an increased generation of prostaglandins in the gastric mucosa[18], we analyzed the effect of prostaglandin PGE2 on PrPc expression in MKN45 cells. At the protein level, we observed a dose-dependent increase in PrPc expression which reached its maximum at the physiological concentration of 10 µmol/L. This could represent another mechanism by which the H pylori-induced inflammatory response triggers protective mechanisms in the gastric mucosa.
According to previous studies, chronic infection with H pylori is accompanied by a significantly increased generation of pro-inflammatory cytokines, especially IL-1β and TNF-α, in the gastric mucosa. Thus, both cytokines could be responsible for the upregulation of PrPc in the gastric mucosa colonized by H pylori. Here we have demonstrated a significant dose-dependent increase of PrPc expression in MKN45 cells incubated with IL-1β, while in contrast, TNF-α showed no effect. We did not further analyze the difference in the action of these two cytokines, but it has been shown by others that these two cytokines evoke different signaling cascades in gastric epithelial cells[31,32].
Gastrin, IL-1β and prostaglandins are not the sole candidates for stimulation of PrPc expression in H pylori infected gastric mucosa. Although not investigated in this study, heat shock proteins could represent additional important factors responsible for the upregulation of PrPc. Previous studies demonstrated that exposure of the gastric mucosa to H pylori lipopolysaccharide leads to a strong upregulation of heat shock proteins which could in turn stimulate the PrPc expression in gastric epithelial cells[33]. In a previous study, it was found that cellular stress upregulates PrPc expression through its interaction with the heat shock elements (HSE) on the PrPc gene promoter[34]. Together these findings suggest a regulation of PrPc expression by heat shock proteins. All these findings demonstrate the complexicity of the regulation PrPc expression and underscore the need to further analyze the precise link between H pylori infection and PrPc expression.
In conclusion, our results indicate that (1) H pylori infection is accompanied by a dramatic upregulation of PrPc expression in the gastric mucosa; (2) this is linked to H pylori-induced hypergastrinemia, increased mucosal prostaglandin synthesis and enhanced mucosal generation of IL-1β; (3) Thus, H pylori infection may promote uptake and propagation of alimentary prions from the gastrointestinal tract by upregulation of PrPc expression.