Published online Dec 28, 2005. doi: 10.3748/wjg.v11.i48.7585
Revised: June 23, 2005
Accepted: June 24, 2005
Published online: December 28, 2005
AIM: To observe the effects of plasma from patients with severe viral hepatitis (SVHP) on the growth and metabolism of porcine hepatocytes and the clinical efficiency of bioartificial liver device.
METHODS: Hepatocytes were isolated from male porcines by collagenase perfusion. The synthesis of DNA and total protein, leakages of AST and LDH, changes in glutathione (GSH), catalase and morphology of porcine hepatocytes exposed to SVHP were investigated to indicate the effect of plasma from patients with severe hepatitis on the growth, injury, detoxification, and morphology of porcine hepatocytes.
RESULTS: The synthesis of DNA and protein was inhibited in the medium containing 100% SVHP compared to the controls. The leakages of LDH and AST increased in porcine hepatocytes following exposure to 100% SVHP for 5 h. The difference between 100% SVHP and 10% newborn calf serum (NCS) was significant in t-test (LDH: t = 24.552, P = 0.001; AST: t = 4.169, P = 0.014). After exposure to SVHP for 24 h, alterations in GSH status were significant (F = 2.746, P<0.05) between porcine hepatocytes in 100% SVHP and 10% NCS, but no alteration occurred in the culture medium after 48 h (F = 4.378, P<0.05). A similar profile was observed in catalase activity. Many round vacuoles were observed in porcine hepatocytes cultured in SVHP. The membranes of these cells became indistinct and almost all the cells died on d 5.
CONCLUSION: Plasma from patients with severe hepatitis inhibits the growth, injures membrane, disturbs GSH homeostasis and induces morphological changes of porcine hepatocytes. It is suggested that SVHP should be pretreated to reduce the toxin load and improve the performance of porcine hepatocytes in extracorporeal liver-support devices.
- Citation: Cheng YB, Wang YJ, Zhang SC, Liu J, Chen Z, Li JJ. Response of porcine hepatocytes in primary culture to plasma from severe viral hepatitis patients. World J Gastroenterol 2005; 11(48): 7585-7590
- URL: https://www.wjgnet.com/1007-9327/full/v11/i48/7585.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i48.7585
A bioartificial liver (BAL) support system, composed of artificial materials and biological components such as hepatocytes, acts as a bridge to provide patients with prolonged time of survival until a donor organ becomes available for the transplantation or their own liver can regenerate[1]. The performance of a BAL depends on the viability and functional activities of hepatocytes in the system. Many laboratories are currently investigating the factors influencing the viability and functional activities of hepatocytes. It has been demonstrated that serum or plasma from liver failure patients interferes extensively with cellular metabolism[2-6]. When the patient’s blood is detoxified by the BAL device, there is contact between the patient’s plasma and cells in the device. It is thus important to assess the direct interactions between plasma and hepatocytes.
Porcine and human hepatocytes have similar physiological characteristics and metabolic functions and are considered to be the best candidate for use in a BAL[7-11]. They have been applied in clinical trials based on their easy source and excellent functions for the synthesis of protein, glucose, and urea as well as lower lactate dehydrogenase release. In vitro, porcine hepatocytes in the bioreactor can clear most conjugated bile acid species from pooled patient plasma[6]. Furthermore, it can be immobilized on a ‘‘hepatocyte/gold colloid’’ interface at which hepatocytes proliferate quickly[12].
In China, HBV infection rate has been estimated to be 10% or higher, and severe hepatitis caused by HBV is common[13,14]. BAL also provides temporary support for these patients when acute or chronic severe viral hepatitis (SVHP) develops. However, there are few reports on how SVHP interferes with the growth and function of hepatocytes in primary culture. Therefore, we investigated the direct interactions between SVHP and porcine hepatocytes.
Cell culture reagents, including RPMI-1640, sodium pyruvate, and L-glutamine were purchased from GIBCO, Life Technologies, Ltd. (Paisley, Scotland, UK). Type IV collagenase was a product of Sigma Chemical Co, Ltd (St. Louis, MO, USA). Reagents for the measurement of reduced glutathione (GSH), catalase (CAT) and total protein (TP) were from Nanjing Biological Technology Co, Ltd. Methyl thiazolyl tetrazolium (MTT) was from Fluka Chemic AG (Switzerland). TriPure isolation reagent was from Roche (Switzerland). Plasticware was from Nunc (Denmark). All solutions were prepared with twice-distilled water.
Healthy Chinese experimental miniature male pigs aged 1-4 d were provided by Experimental Animal Center of Third Military Medical University. The research protocol was in compliance with Chinese guidelines for the humane care of experimental animals. Porcine hepatocytes were isolated by modified two-step in situ collagenase perfusion method[15,16]. The viability of freshly isolated suspensions determined by trypan blue exclusion was 85-95%. Porcine hepatocytes were cultured at 37 °C for 24 h in RPMI-1640 medium supplemented with 10% (vol/vol) newborn calf serum (NCS) at a density of 2×105 cells/mL in a 50 mL/L CO2 incubator. The cultures were washed twice in warm phosphate-buffered saline (PBS) and cultured in a medium containing 10% (vol/vol) NCS, normal plasma (NP) anti-coagulated by heparin, and SVHP. Porcine hepatocytes were prepared for assay as described below.
Plasma was obtained from six patients with SVHP (3 females, 3 males, aged 34-60 years) at the onset of plasmapheresis and stored at -80 °C until use. Diagnosis of these patients was in accordance with the criteria of severe hepatitis described in the Viral Hepatitis Protection and Cure Guideline established by the Chinese Infection and Hepatology Association. In these six patients, total bilirubin (TB) averaged 611.8 μmol/L, prothrombin time (PT) averaged 32 s, total bile acids (TBA) averaged 309.8 μmol/L and PTa averaged 29%. Hepatitis B surface antigen (HBsAg) was positive and HBV-DNA was greater than 105 copies/mL in all the 6 patients. All patients suffered from hepatic encephalopathy, grade II in two patients, grade III in three patients, and grade IV in one patient. Normal serum was obtained from normal individuals.
Cells were seeded in 24-well plates in 500 μL medium. The viability was assessed by tetrazolium bromide assay (MTT) on d 0-5 after exposure to the culture medium containing 10% (vol/vol) NCS, 100% NP, and 100% SVHP.
After being cultured in six-well plates for 24 h, the media were discarded, and DNA was isolated as the procedures of TriPure isolation reagent description. DNA content was determined using a spectrophotometer (SmartSpec 3000, BioRad, USA).
After being incubated for 24 and 48 h, monolayer cells were washed and dissolved. Total cellular protein was digested in 0.5 mol/L NaOH and measured by micromodification as previously described[17].
Porcine hepatocytes were exposed to 100% SVHP and 10% (vol/vol) NCS in RPMI-1640 for 5 h. The media were washed thrice with PBS and replaced with RPMI-1640 without plasma and serum. After 24 h of culture, the leakage of LDH and AST from hepatocytes into the supernatant was measured using an automated chemical analyzer (Model 7020 Hitachi Co., Tokyo, Japan)[18].
After being incubated for 24 and 48 h, the medium containing 10% NCS, 100% NP, 100% SVHP was removed, the wells were washed with PBS, and GSH was added into 0.2 mL 10% (w/v) trichloroacetic acid for 10 min at room temperature. Samples were frozen at -20 °C until measurement of GSH by fluorimetry. CAT activity was measured.
Cultured hepatocytes were observed daily under phase contrast microscope (IX70, Olympus, Tokyo, Japan), and the morphological changes were compared.
Data were expressed as mean±SD. Statistical analysis was carried out by analysis of variance and t-test. P<0.05 was considered statistically significant.
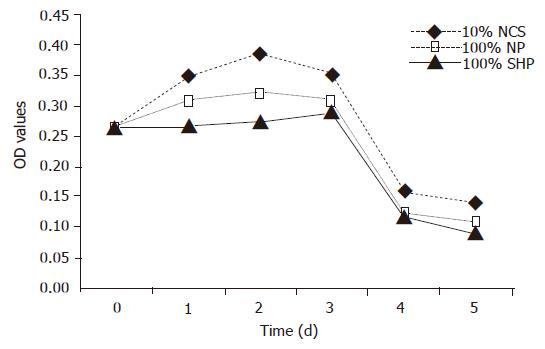
The viability of porcine hepatocytes cultured in 100% SVHP was significantly lower than that cultured in the medium containing 10% NCS (F = 6.328, P<0.01). The viability of porcine hepatocytes in 100% NP group was not higher than that in 10% NCS group. The viability of porcine hepatocytes in all the groups tended to decrease from the third day (Figure 1).
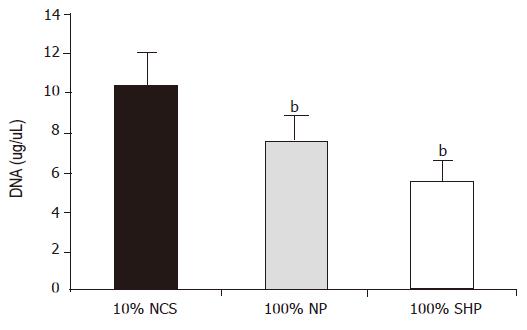
After being cultured for 24 h, the DNA level in three different media was significantly different (F = 20.107, P<0.01). The level was the lowest in 100% SVHP and lower in 100% NP than in 10% NCS. The inhibitory effect of porcine hepatocytes on DNA synthesis is shown in Figure 2.
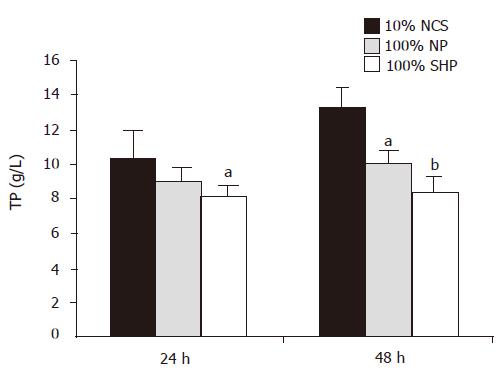
During the course of culture, the amount of TP in three different media was significantly different (F = 9.281, P<0.01, Figure 3). Multiple comparison showed that the TP level in 100% SVHP was lower than that in 10% NCS (P<0.01). The TP level in 100% NP was also significantly lower than that in 10% NCS (P<0.05).
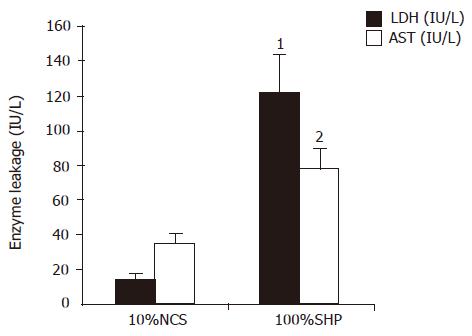
LDH and AST elevations were observed in 100% SVHP group after 5 h of culture. The LDH level in 100% SVHP group was significantly higher than that in 10% NCS group (t = 24.552, P = 0.001). The AST level in 100% SVHP group was also significantly higher than that in 10% NCS group (t = 4.169, P = 0.014, Figure 4), indicating that hepatocytes cultured in plasma from patients with SVHP had damage in the cell membrane.
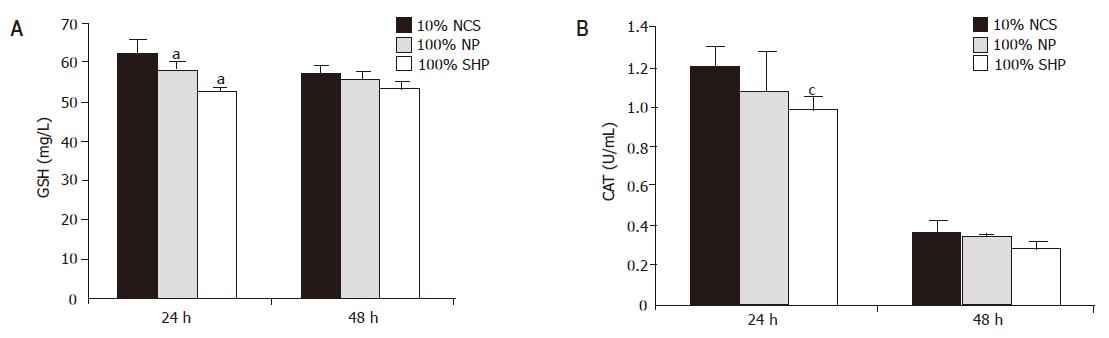
GSH concentration in porcine hepatocytes decreased in SVHP and NP as compared to that in the culture medium containing 10% NCS (Figure 5A). A significant decrease in GSH content was observed in SVHP compared to that in the medium containing 10% NCS within 24 h (F = 2.746, P<0.05). After 48 h the GSH level declined slightly. There was no difference between 100% SVHP and 10% NCS (F = 4.378, P>0.05). A similar profile was observed in CAT levels following incubation with SVHP and NP (Figure 5B). The only difference between GSH and CAT was that the CAT content was dramatically decreased after being cultured for 48 h.
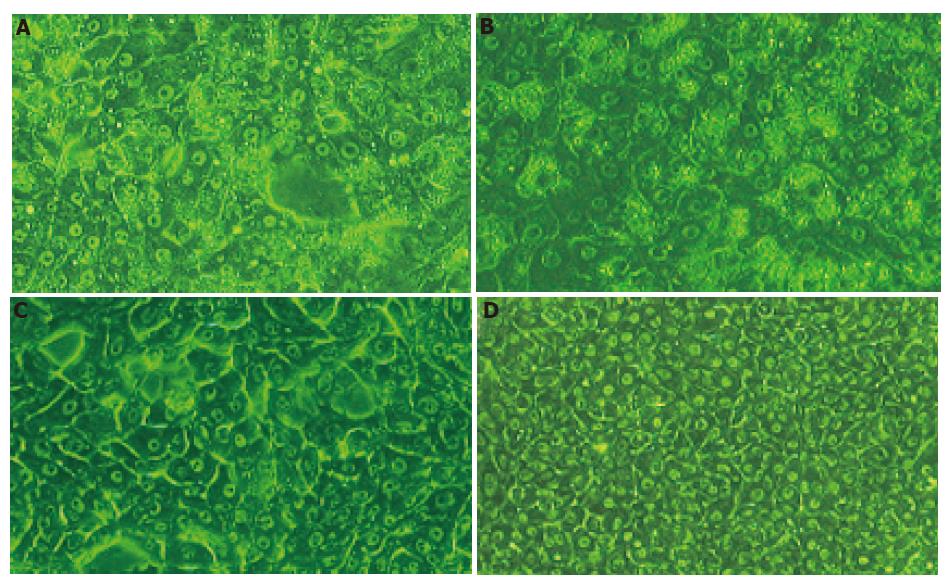
The dramatic change of porcine hepatocyte morphology after 24 h exposure to 100% SVHP was observed under phase contrast microscope. As shown in Figures 6A-D, most of the cells became detached and deformed. Vacuolization in cytoplasm and necrosis were more commonly observed. Membranes of the cells became indistinct. These morphological changes became marked after 48 h of culture. Vacuoles were mainly concentrated around cell nuclei and detached from their dishes. During the 48-h culture, hepatocytes in groups of 100% NP and 10% NCS spread and formed a clear confluent monolayer. Detachment, deformity, and vacuolization were not observed in the cytoplasm. There was no obvious difference between the two groups. After 5 d of culture, necrosis was observed in the two groups and almost all hepatocytes were dead in 100% SVHP group.
Experimental studies[3-5] have demonstrated that plasma from patients with fulminant hepatic failure (FHF) or liver failure (LF) can inhibit the growth of hepatocytes and synthesis of macromolecules in hepatocytes. McCloskey et al[5]. found that plasma from patients with liver failure could diminish DNA and protein synthesis. Shi et al[4]. reported that serum from patients with FHF inhibits the growth rate and synthesis of DNA, RNA, and protein. In our study, the plasma was from patients with SVHP. Inhibition of growth and macromolecule synthesis, reduction of viability of porcine hepatocytes were demonstrated. The inhibition mechanism might be related to endogenic materials such as bile acids, growth factors, and cytokines in SVHP. There are more bile acids, hepatic growth factor (HGF) and tumor growth factor-β (TGF-β)[19] in SVHP. Accumulation of intracellular bile acids causes damage to intracellular organelles, such as mitochondria[20]. Bile acids in nuclei cause DNA injury by changing chromatin structure or by activating nuclear nuclease, thus resulting in conversion into a metabolite that could directly damage DNA[21,22]. HGF does not always accelerate the growth of hepatocytes. Sometimes there are converse results[23]. It was reported that HGF inhibits cell growth at a cell cycle phase and growth of cells is impaired with the increasing HGF concentration, while elevated serum TGF may lead to an increase in p21, a cyclin-dependent kinase inhibitor that can inhibit the activation of G1-associated cyclins such as Cdk2[24,25]. In this study, the viability of porcine hepatocytes cultured in 100% NP was decreased compared to that in 10% NCS, which may result from cytokine- and antibody-mediated damage.
Membrane damage and enzyme leakage have been detected in the culture medium[5,26,27]. McCloskey et al[5]. reported that after HHY41 hepatocytes are exposed to plasma from patients with LF containing Na251CrO4 for 16 h, the leakage of 51Cr is about 20 times higher than that in controls. However, Uchino et al[28]. demonstrated that the function of pig hepatocytes does not deteriorate when they are exposed to the serum from the patients with FHF[28]. In this study, LDH and AST leakage from porcine hepatocytes cultured in SVHP was significantly higher than that from porcine hepatocytes cultured in 10% NCS. Morphological changes of hepatocytes also suggested severe cell membrane damage, indicating that SVHP can lead to hepatocellular membrane impairment. Endogenic toxins may originate either from cellular necrosis debris and breakdown products released into the blood stream or from accumulation of an agent that is normally eliminated by metabolism in hepatocytes. Bile acids play an important role in the toxicities and can form micelles with cholesterol and phospholipids on the surface of hepatocyte membranes, which produce apparent hepatocellular membrane injury[22]. Virus-mediated damage involves immune-mediated and direct viral cytopathic effects.
The highest cytotoxicity values have been found in the plasma from patients with FHF, indicating the utmost importance of detoxification by BAL. Intracellular GSH content often reflects the fate of potentially harmful substances, and examination of GSH status may provide information on the mechanisms of toxicity. Previous experimental studies revealed that exposure of hepatocytes to the serum from patient with FHF results in increase or decrease in GSH content. The changes are related to plasma concentration, culture time, hepatocyte type[4,5]. Previous studies have shown that Hep G2 cells respond to abnormal culture conditions and toxic concentrations of bile. GSH of HHY41 in plasma from patients with LF is decreased[5]. In our study, the pronounced decrease in GSH levels was observed following incubation with SVHP. As a main member of anti-oxidizing system, GSH plays an important role in relieving the tissue depression due to electrophilic reactive toxins or oxidative stress because it can react directly with reactive oxygen free-radicals and conjugate bile acids[29].These factors lead to a depletion of cellular GSH store. The activity of CAT changes as GSH, which requires further investigation.
Science Editors Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Hui T, Rozga J, Demetriou AA. Bioartificial liver support. J Hepatobiliary Pancreat Surg. 2001;8:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Hughes RD, Cochrane AM, Thomson AD, Murray-Lyon IM, Williams R. The cytotoxicity of plasma from patients with acute hepatic failure to isolated rabbit hepatocytes. Br J Exp Pathol. 1976;57:348-353. [PubMed] |
| 3. | Gove CD, Hughes RD, Williams R. Rapid inhibition of DNA synthesis in hepatocytes from regenerating rat liver by serum from patients with fulminant hepatic failure. Br J Exp Pathol. 1982;63:547-553. [PubMed] |
| 4. | Shi Q, Gaylor JD, Cousins R, Plevris J, Hayes PC, Grant MH. The effects of serum from patients with acute liver failure on the growth and metabolism of Hep G2 cells. Artif Organs. 1998;22:1023-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | McCloskey P, Tootle R, Selden C, Larsen F, Roberts E, Hodgson HJ. Modulation of hepatocyte function in an immortalized human hepatocyte cell line following exposure to liver-failure plasma. Artif Organs. 2002;26:340-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Pazzi P, Morsiani E, Vilei MT, Granato A, Rozga J, Demetriou AA, Muraca M. Serum bile acids in patients with liver failure supported with a bioartificial liver. Aliment Pharmacol Ther. 2002;16:1547-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Rozga J, Williams F, Ro MS, Neuzil DF, Giorgio TD, Backfisch G, Moscioni AD, Hakim R, Demetriou AA. Development of a bioartificial liver: properties and function of a hollow-fiber module inoculated with liver cells. Hepatology. 1993;17:258-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 184] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 8. | Watanabe FD, Mullon CJ, Hewitt WR, Arkadopoulos N, Kahaku E, Eguchi S, Khalili T, Arnaout W, Shackleton CR, Rozga J. Clinical experience with a bioartificial liver in the treatment of severe liver failure. A phase I clinical trial. Ann Surg. 1997;225:484-491; discussion 491-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 320] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 9. | Khalili TM, Navarro A, Ting P, Kamohara Y, Arkadopoulos N, Solomon BA, Demetriou AA, Rozga J. Bioartificial liver treatment prolongs survival and lowers intracranial pressure in pigs with fulminant hepatic failure. Artif Organs. 2001;25:566-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Nagaki M, Miki K, Kim YI, Ishiyama H, Hirahara I, Takahashi H, Sugiyama A, Muto Y, Moriwaki H. Development and characterization of a hybrid bioartificial liver using primary hepatocytes entrapped in a basement membrane matrix. Dig Dis Sci. 2001;46:1046-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Mears DC, Stewart G, Sun J, Woodman K, Bourne R, Wang L, Sheil AG. Experience with a porcine hepatocyte-based bioartificial liver support system. Transplant Proc. 2003;35:441-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Gu HY, Chen Z, Sa RX, Yuan SS, Chen HY, Ding YT, Yu AM. The immobilization of hepatocytes on 24 nm-sized gold colloid for enhanced hepatocytes proliferation. Biomaterials. 2004;25:3445-3451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Merican I, Guan R, Amarapuka D, Alexander MJ, Chutaputti A, Chien RN, Hasnian SS, Leung N, Lesmana L, Phiet PH. Chronic hepatitis B virus infection in Asian countries. J Gastroenterol Hepatol. 2000;15:1356-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 343] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 14. | Pokorski RJ, Ohlmer U. Long-term morbidity and mortality in Chinese insurance applicants infected with the hepatitis B virus. J Insur Med. 2001;33:143-164. [PubMed] |
| 15. | Chen Z, Ding Y, Zhang H. Cryopreservation of suckling pig hepatocytes. Ann Clin Lab Sci. 2001;31:391-398. [PubMed] |
| 16. | Wang YJ, Liu HL. Mass isolation and cryopreservation of hepatocytes. Bioartificial Liver. Beijing-China: People's Health Publishing House 2000; 207-2009. |
| 17. | LOWRY OH, ROSEBROUGH NJ, FARR AL, RANDALL RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265-275. [PubMed] |
| 18. | Flendrig LM, la Soe JW, Jörning GG, Steenbeek A, Karlsen OT, Bovée WM, Ladiges NC, te Velde AA, Chamuleau RA. In vitro evaluation of a novel bioreactor based on an integral oxygenator and a spirally wound nonwoven polyester matrix for hepatocyte culture as small aggregates. J Hepatol. 1997;26:1379-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 162] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 19. | Nozato E, Shiraishi M, Nishimaki T. Up-regulation of hepatocyte growth factor caused by an over-expression of transforming growth factor beta, in the rat model of fulminant hepatic failure. J Surg Res. 2003;115:226-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Hoshino M, Ohiwa T, Hayakawa T, Kamiya Y, Tanaka A, Hirano A, Kumai T, Katagiri K, Miyaji M, Takeuchi T. Effects of dibutyryl cyclic AMP and papaverine on intrahepatocytic bile acid transport. Role of vesicle transport. Scand J Gastroenterol. 1993;28:833-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Russo P, Taningher M, Pala M, Pisano V, Pedemonte P, De Angeli MT, Carlone S, Santi L, Parodi S. Characterization of the effects induced on DNA in mouse and hamster cells by lithocholic acid. Cancer Res. 1987;47:2866-2874. [PubMed] |
| 22. | Kulkarni MS, Cox BA, Yielding KL. Requirements for induction of DNA strand breaks by lithocholic acid. Cancer Res. 1982;42:2792-2795. [PubMed] |
| 23. | Drixler TA, Vogten MJ, Ritchie ED, van Vroonhoven TJ, Gebbink MF, Voest EE, Borel Rinkes IH. Liver regeneration is an angiogenesis- associated phenomenon. Ann Surg. 2002;236:703-11; discussion 711-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 107] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 24. | Albrecht JH, Meyer AH, Hu MY. Regulation of cyclin-dependent kinase inhibitor p21(WAF1/Cip1/Sdi1) gene expression in hepatic regeneration. Hepatology. 1997;25:557-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Sugiyama A, Nagaki M, Shidoji Y, Moriwaki H, Muto Y. Regulation of cell cycle-related genes in rat hepatocytes by transforming growth factor beta1. Biochem Biophys Res Commun. 1997;238:539-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Ohiwa T, Katagiri K, Hoshino M, Hayakawa T, Nakai T. Tauroursodeoxycholate and tauro-beta-muricholate exert cytoprotection by reducing intrahepatocyte taurochenodeoxycholate content. Hepatology. 1993;17:470-476. [PubMed] |
| 27. | Heuman DM, Pandak WM, Hylemon PB, Vlahcevic ZR. Conjugates of ursodeoxycholate protect against cytotoxicity of more hydrophobic bile salts: in vitro studies in rat hepatocytes and human erythrocytes. Hepatology. 1991;14:920-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 161] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 28. | Uchino J, Matsue H, Takahashi M, Nakajima Y, Matsushita M, Hamada T, Hashimura E. A hybrid artificial liver system. Function of cultured monolayer pig hepatocytes in plasma from hepatic failure patients. ASAIO Trans. 1991;37:M337-M338. [PubMed] |
| 29. | Smirthwaite AD, Gaylor JD, Cousins RB, Grant MH. Cytotoxicity of bile in human Hep G2 cells and in primary cultures of rat hepatocytes. Artif Organs. 1998;22:831-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |