Published online Dec 21, 2005. doi: 10.3748/wjg.v11.i47.7457
Revised: August 13, 2005
Accepted: August 17, 2005
Published online: December 21, 2005
AIM: To investigate the release of cyclodextrin-5-aminosalicylic acid (CyD-5-ASA) in cecum and colon.
METHODS: An anti-inflammatory drug 5-ASA was conjugated onto the hydroxyl groups of α-, β- and γ-cyclodextrins (CyDs) through an ester linkage, and the in vivo drug release behavior of these prodrugs in rat’s gastrointestinal tract after the oral administration was investigated.
RESULTS: The 5-ASA concentration in the rat’s stomach and small intestine after the oral administration of CyD-5-ASA conjugate was much lower than that after the oral administration of 5-ASA alone. The lower concentration was attributable to the passage of the conjugate through the stomach and small intestine without significant degradation or absorption, followed by the degradation of the conjugate site-specific in the cecum and colon. The oral administration of CyD-5-ASA resulted in lower plasma and urine concentration of 5-ASA than that of 5-ASA alone.
CONCLUSION: CyD-5-ASA conjugates may be used as prodrugs for colon-specific drug delivery system.
- Citation: Zou MJ, Cheng G, Okamoto H, Hao XH, An F, Cui FD, Danjo K. Colon-specific drug delivery systems based on cyclodextrin prodrugs: In vivo evaluation of 5-aminosalicylic acid from its cyclodextrin conjugates. World J Gastroenterol 2005; 11(47): 7457-7460
- URL: https://www.wjgnet.com/1007-9327/full/v11/i47/7457.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i47.7457
Ulcerative colitis and Crohn’s disease are recurrent disorders chronically involving the mucosa and sub-mucosa of the colon. 5-Aminosalicylic acid (5-ASA) is an active ingredient of agents used for the long-term maintenance therapy to prevent relapses of Crohn’s disease and ulcerative colitis[1-3]. However, when 5-ASA is administered orally, a large amount of the drug is absorbed from the upper gastrointestinal tract (GIT), and causes systemic side effects. Therefore, it is preferable to deliver the drug site-specifically to the colon. Various approaches have been developed, including coating with biodegradable polymers[4,5], coating with pH-sensitive polymers[6,7], time-dependent formulations[8,9], forming biodegradable matrices[10,11], and forming prodrugs.
Azo prodrugs of 5-ASA are the most effective prodrugs used for the treatment of inflammatory bowel disease (IBD). Polymeric prodrugs of 5-ASA have not been used in clinics[12,13]. Natural polysaccharides have been used as tools to deliver the drugs to the colon. These polysaccharides remain intact in the physiological environment of the stomach and small intestine, but once the dosage form enters into the colon, it is acted upon by polysaccharides, which release the drug into the colon[14-16].
Cyclodextrins are cyclic oligosaccharides that consists of 6-8 glucose units. They are known to be barely capable of being hydrolyzed and only slightly absorbed in the passage through the stomach and small intestine, and are fermented by colonic microflora into small saccharides. The present study aimed to clarify the in vivo release behavior of 5-ASA from its CyD ester prodrugs in rat’s intestines.
α-, β-, γ-CyDs and carbonyldiimidazole (CDI) were obtained from Sigma Chemical Co (St. Louis, USA). 5-ASA was purchased from Acros Organics Ltd (NJ, USA). CyD-5-ASA and Ac-5-ASA were prepared according to the method reported in a previous paper[17]. All other chemicals and solvents were of analytical reagent grade, and deionized double-distilled water was used throughout the study.
The HPLC system that consisted of LC-10ADvp pumps, SPD-10ADvp detector, and SIL-10ADvp autoinjector was purchased from Shimadzu. Reversed-phase HPLC conditions[18,19] for the determination of 5-ASA and its metabolites were as follows: a mobile phase of 5.0 mmol/L pH 6.0 phosphate buffer/acetonitrile/ 0.1 mol/L tetrabutyl-ammonium chloride (90:10:0.5 v/v/v), a flow rate of 1.0 mL/min, and a detection of wavelength 330 nm for 0-9 min, 240 nm after 9 min.
Male SD rats, weighing about 200 g, were fasted for 8 h prior to drug administration while water was allowed ad libitum. 5-ASA or CyD-5-ASA (equivalent to 50 mg of 5-ASA) was orally administered to the rats and then the rats were placed in metabolic cages. At appropriate intervals (4, 8, 12, and 24 h), blood samples (about 3 mL) were taken from the jugular vein, and the rats were killed by ether anesthesia, followed by thoracotomy. The blood was centrifuged at 10 000 g for 5 min, and serum was frozen at -20 °C and stored until analysis. The GIT was isolated and divided into stomach, small intestine, cecum, and colon. The intestinal contents were removed by gently squeezing the gastrointestinal segments, which were then rinsed with a small amount of phosphate buffer (about 10 mL) to remove the adherent materials. The separated contents and tissues were quickly frozen at -20 °C and stored until analysis. Urine and feces samples were collected and frozen at -20 °C.
The frozen intestinal contents were thawed, weighed, and diluted with 1.5 mL of 0.1 mol/L HCl. They were vortexed for 3 min, centrifuged at 5 000 r/min for 5 min, then 2.5 mL of methanol was added to 0.8 mL of the supernatant, vortexed for 2 min. The mixture was centrifuged for 10 min at 5 000 r/min. The supernatant of 3.0 mL was transferred into another cleaning tube and evaporated under N2 at 40 °C. The remaining supernatant was dissolved in mobile phase and filtered through 0.2-mL membrane. The frozen tissues were thawed, weighed, cut into small pieces and homogenized with five volumes of cold 0.1 mol/L HCl using a tissue homogenizer (Polytron PT-MR3100, Kinematica, Switzerland) at 0 °C.
In a previous paper[17], we have reported that the hydroxyl propyl cellulose/5-ASA (HPC-5-ASA), chitosan/5-ASA (ChT-5-ASA) conjugate are stable in rat’s GIT, where CyD-5-ASA liberates 5-ASA site-specifically in rat’s cecum and colon. In this study, the distribution of CyD-5-ASA prodrugs, 5-ASA and its metabolites were investigated.
Table 1 and Table 2, Table 3 show the recovery percentage of the intact prodrugs, 5-ASA and N-acetyl-5-ASA (Ac-5-ASA), a metabolite of 5-ASA in GIT, blood, liver, feces, and urine after the oral administration of CyD-5-ASA to rats. The sampling time was chosen on the basis of the time necessary for CyD-5-ASA to reach the cecum after oral administration. The total recovery was defined as the sum of the intact prodrugs, 5-ASA and Ac-5-ASA in the entire GIT and tissues, blood (assuming that the blood volume is 6.5% of body weight), urine and feces, and expressed as a percentage of the dose administered. The recovery from the stomach, small intestine, cecum, colon, blood, urine, feces, and liver was expressed as a percentage of the total recovery described above.
| Time | Stomach | Small intestine | Cecum | Colon | |||||||||
| (h) | Prodrug | 5-ASA | AC-5-ASA | Prodrug | 5-ASA | AC-5-ASA | Prodrug | 5-ASA | AC-5-ASA | Prodrug | 5-ASA | AC-5-ASA | |
| 4 | 19.8±4.8 | 0.0±0.0 | 0.0±0.0 | 41.6±30.8 | 0.0±0.0 | 0.0±0.0 | 7.7±7.6 | 14.7±17.3 | 7.3±12.6 | 6.3±11.0 | 0.8±1.0 | 1.8±1.6 | |
| 8 | 2.8±4.3 | 0.0±0.0 | 0.0±0.0 | 14.3±6.5 | 0.7±0.4 | 0.0±0.0 | 11.3±14.0 | 20.2±22.4 | 6.1±5.8 | 7.2±9.9 | 9.1±7.7 | 18.4±13.6 | |
| 12 | 0.0±0.0 | 0.1±0.1 | 0.4±0.5 | 1.7±0.4 | 0.5±0.7 | 2.5±3.9 | 9.4±11.8 | 4.7±7.4 | 6.2±6.4 | 17.2±24.0 | 17.3±23.7 | 16.6±17.9 | |
| 24 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 22.8±24.2 | 9.2±9.0 | 0.3±0.5 | 7.9±13.6 | 13.3±15.6 | 1.4±1.1 | 23.4±15.6 | |
| Plasma | Urine | Feces | Liver | Total | |||||||||
| Prodrug | 5-ASA | AC-5-ASA | Prodrug | 5-ASA | AC-5-ASA | Prodrug | 5-ASA | AC-5-ASA | Prodrug | 5-ASA | AC-5-ASA | recovery | |
| 4 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 69.3±29.0 |
| 8 | 0.0±0.0 | 0.5±0.3 | 4.0±2.6 | 0.0±0.0 | 0.2±0.1 | 2.0±1.3 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.3±0.1 | 3.0±2.2 | 65.0±11.9 |
| 12 | 0.0±0.0 | 0.4±0.5 | 4.7±4.0 | 0.0±0.0 | 0.3±0.4 | 2.8±2.4 | 0.3±0.5 | 7.1±8.2 | 7.1±8.0 | 0.0±0.0 | 0.2±0.3 | 0.4±0.8 | 50.5±20.0 |
| 24 | 0.0±0.0 | 1.4±1.2 | 1.9±1.9 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 4.3±7.4 | 1.3±1.3 | 9.3±11.8 | 0.0±0.0 | 1.4±1.3 | 2.1±2.5 | 12.6±7.6 |
| Time | Stomach | Small intestine | Cecum | Colon | |||||||||
| (h) | Prodrug | 5-ASA | AC-5-ASA | Prodrug | 5-ASA | AC-5-ASA | Prodrug | 5-ASA | AC-5-ASA | Prodrug | 5-ASA | AC-5-ASA | |
| 4 | 26.7±3.2 | 0.0±0.0 | 0.0±0.0 | 47.0±27.6 | 0.0±0.0 | 0.0±0.0 | 13.2±13.7 | 1.0±0.6 | 0.3±0.3 | 9.7±16.9 | 1.2±1.4 | 0.9±1.4 | |
| 8 | 8.5±12.9 | 0.0±0.0 | 0.0±0.0 | 14.9±6.6 | 2.4±3.4 | 0.0±0.0 | 30.6±35.8 | 7.5±11.6 | 3.8±3.8 | 12.3±17.0 | 9.1±4.4 | 4.3±2.9 | |
| 12 | 0.0±0.0 | 0.0±0.0 | 0.7±1.2 | 6.6±7.9 | 0.4±0.3 | 3.0±0.8 | 8.4±7.4 | 18.7±13.1 | 11.7±12.8 | 16.1±12.6 | 13.5±12.4 | 7.9±7.3 | |
| 24 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 2.9±4.4 | 16.6±16.1 | 10.3±16.7 | 17.4±15.2 | 27.2±45.2 | 11.0±12.9 | 5.3±5.9 | |
| Plasma | Urine | Feces | Liver | Total | |||||||||
| Prodrug | 5-ASA | AC-5-ASA | Prodrug | 5-ASA | AC-5-ASA | Prodrug | 5-ASA | AC-5-ASA | Prodrug | 5-ASA | AC-5-ASA | recovery | |
| 4 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 64.0±14.3 |
| 8 | 0.0±0.0 | 0.7±0.7 | 2.4±3.0 | 0.0±0.0 | 0.1±0.0 | 0.4±0.4 | 0.0±0.0 | 0.1±0.0 | 0.1±0.1 | 0.0±0.0 | 1.9±2.5 | 1.1±1.6 | 40.6±14.7 |
| 12 | 0.0±0.0 | 1.2±0.5 | 4.1±4.6 | 0.0±0.0 | 2.4±3.5 | 2.3±2.2 | 0.3±0.9 | 0.0±0.0 | 1.1±1.8 | 0.0±0.0 | 0.5±0.7 | 0.8±1.2 | 35.6±10.7 |
| 24 | 0.0±0.0 | 2.3±2.6 | 1.7±2.2 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 4.6±7.9 | 0.2±0.2 | 0.7±0.9 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 14.8±6.5 |
| Time | Stomach | Small intestine | Cecum | Colon | |||||||||
| (h) | Prodrug | 5-ASA | AC-5-ASA | Prodrug | 5-ASA | AC-5-ASA | Prodrug | 5-ASA | AC-5-ASA | Prodrug | 5-ASA | AC-5-ASA | |
| 4 | 19.8±9.4 | 0.0±0.0 | 0.0±0.0 | 46.4±28.7 | 0.2±0.3 | 0.0±0.0 | 10.0±10.1 | 0.9±0.3 | 5.4±4.8 | 8.4±14.6 | 6.4±5.4 | 2.2±1.8 | |
| 8 | 3.2±5.0 | 0.0±0.0 | 0.0±0.0 | 16.2±3.3 | 2.4±1.5 | 0.0±0.0 | 18.2±24.8 | 15.7±9.4 | 2.2±2.7 | 4.0±4.2 | 20.2±8.2 | 7.7±4.2 | |
| 12 | 0.0±0.0 | 0.2±0.0 | 0.6±0.6 | 2.1±1.2 | 0.8±0.3 | 0.0±0.0 | 7.6±7.0 | 4.9±2.5 | 14.1±12.2 | 7.9±5.2 | 7.2±1.5 | 34.6±20.8 | |
| 24 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.8±0.7 | 5.3±5.8 | 2.1±1.8 | 40.5±35.4 | 15.0±22.3 | 19.4±15.6 | 5.1±4.0 | |
| Plasma | Urine | Feces | Liver | Total | |||||||||
| Prodrug | 5-ASA | AC-5-ASA | Prodrug | 5-ASA | AC-5-ASA | Prodrug | 5-ASA | AC-5-ASA | Prodrug | 5-ASA | AC-5-ASA | recovery | |
| 4 | 0.0±0.0 | 0.0±0.0 | 0.2±0.2 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 76.8±12.2 |
| 8 | 0.0±0.0 | 0.2±0.1 | 2.5±2.4 | 0.0±0.0 | 1.1±0.2 | 1.8±1.8 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 2.9±2.2 | 1.7±1.8 | 61.9±22.0 |
| 12 | 0.0±0.0 | 1.8±1.7 | 3.8±3.5 | 0.0±0.0 | 0.1±0.0 | 6.4±6.6 | 0.5±0.8 | 2.2±0.8 | 0.0±0.0 | 0.0±0.0 | 0.9±0.6 | 4.3±0.2 | 48.5±6.8 |
| 24 | 0.0±0.0 | 1.3±1.4 | 0.6±0.1 | 0.0±0.0 | 0.1±0.0 | 2.5±2.4 | 3.7±4.1 | 0.3±0.0 | 3.4±1.3 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 17.2±2.4 |
Four hours after dosing, about 60% of 5-ASA and Ac-5-ASA were recovered in intact form from the stomach and small intestine and about 40% in the cecum and colon. Eight hours after dosing, 20% of the prodrugs were located in the stomach and small intestine and a large portion (>70%) was recovered from cecum and colon. After 12 h, the major portion of the prodrugs was recovered from the cecum, colon, and feces. There was only a negligible amount of 5-ASA and Ac-5-ASA in blood and urine. These results indicated that the CyD prodrugs survived passage through the rat’s stomach and small intestine and were subjected to the ring-opening process in the cecum and colon.
In this study, 5-ASA, α-CyD-5-ASA, β-CyD-5-ASA and γ-CyD-5-ASA (equivalent to 50 mg/kg 5-ASA) were administered to the rats. There was no or a very small amount of 5-ASA detected in the tissues after the oral administration. The results of β-CyD-5-ASA and γ-CyD-5-ASA were similar to those of α-CyD-5-ASA.
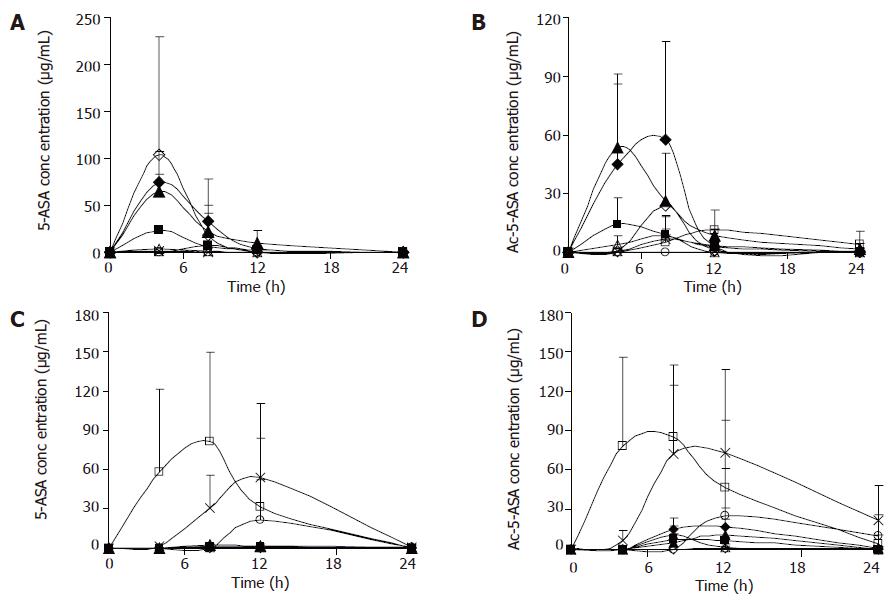
Figure 1 shows the concentrations of 5-ASA and Ac-5-ASA in rat’s blood, urine, feces, liver homogenates after the oral administration of 5-ASA (50 mg/kg) and α-CyD-5-ASA (equivalent to 50 mg/kg 5-ASA). With the oral administration of 5-ASA, 5-ASA being absorbed in the upper GIT, the concentration in cecum and colon was very low. Whereas during the oral administration of α-CyD-5-ASA, the levels of 5-ASA and Ac-5-ASA in the plasma and urine were very low in the whole experimental period, which might indicate the limited oral absorption of α-CyD-5-ASA. α-CyD-5-ASA was not degraded to release 5-ASA in the GIT, and 5-ASA was detected in the cecum and colon. A portion of the released 5-ASA might be absorbed in the large intestine and appeared in the plasma concentration of Ac-5-ASA.
In this study, formation of prodrugs has improved the delivery properties of the parent drug molecule. Activated by the microbial enzymes in the large intestine, CyD-5-ASA released 5-ASA at a controlled rate. These results suggest that after the oral administration of prodrugs there is no or a very small amount of 5-ASA in the tissues of rats. Oral administration of 5-ASA gives much higher plasma and urine concentrations of 5-ASA than CyD-5-ASA and the conjugate can lower the plasma concentration of 5-ASA. The lower concentration is attributable to the passage of the conjugate through the stomach and small intestine without significant degradation or absorption, followed by the degradation of the conjugate site-specifically in the cecum and colon. Therefore, CyD-5-ASA conjugates may be useful as prodrugs for colon-specific delivery system.
Co-first-authors: Mei-Juan Zou and Gang Cheng
Science Editor Wang XL Language Editor Elsevier HK
| 1. | Schacht E, Gevaert A, Kenawy ER, Molly K, Verstraete W, Adriaensens P, Caleer R, Gelan J. Polymers for colon specific drug delivery. J Control Release. 1996;39:327-338 DOI : 10.1016/0168-3659(95)00184-0. |
| 2. | Carceller E, Salas J, Merlos M, Giral M, Ferrando R, Escamilla I, Ramis J, García-Rafanell J, Forn J. Novel azo derivatives as prodrugs of 5-aminosalicylic acid and amino derivatives with potent platelet activating factor antagonist activity. J Med Chem. 2001;44:3001-3013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Brown JP, McGarraugh GV, Parkinson TM, Wingard RE, Onderdonk AB. A polymeric drug for treatment of inflammatory bowel disease. J Med Chem. 1983;26:1300-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 106] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Milojevic S, Newton JM, Cummings JH, Gibson GR, Botham RL, Ring SG, Stockham M, Allwood MC. Amylose as a coating for drug delivery to the colon: Preparation and in vitro evaluation using glucose pellets. J Control Release. 1996;38:85-94 DOI : 10.1016/0168-3659(95)00113-1. |
| 5. | Van den Mooter G, Maris B, Samyn C, Augustijns P, Kinget R. Use of azo polymers for colon-specific drug delivery. J Pharm Sci. 1997;86:1321-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 81] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Khan MZ, Stedul HP, Kurjaković N. A pH-dependent colon-targeted oral drug delivery system using methacrylic acid copolymers. II. Manipulation of drug release using Eudragit L100 and Eudragit S100 combinations. Drug Dev Ind Pharm. 2000;26:549-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Wilding IR, Hardy JG, Sparrow RA, Davis SS, Daly PB, English JR. In vivo evaluation of enteric-coated naproxen tablets using gamma scintigraphy. Pharm Res. 1992;9:1436-1441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Fukui E, Miyamura N, Uemura K, Kobayashi M. Preparation of enteric coated timed-release press-coated tablets and evaluation of their function by in vitro and in vivo tests for colon targeting. Int J Pharm. 2000;204:7-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Gupta VK, Beckert TE, Price JC. A novel pH- and time-based multi-unit potential colonic drug delivery system. I. Development. Int J Pharm. 2001;213:83-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 110] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Siew LF, Man SM, Newton JM, Basit AW. Amylose formulations for drug delivery to the colon: a comparison of two fermentation models to assess colonic targeting performance in vitro. Int J Pharm. 2004;273:129-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Zambito Y, Di Colo G. Preparation and in vitro evaluation of chitosan matrices for colonic controlled drug delivery. J Pharm Pharm Sci. 2003;6:274-281. [PubMed] |
| 12. | Sinha VR, Kumria R. Polysaccharide matrices for microbially triggered drug delivery to the colon. Drug Dev Ind Pharm. 2004;30:143-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Jung YJ, Lee JS, Kim YM. Colon-specific prodrugs of 5-aminosalicylic acid: synthesis and in vitro/in vivo properties of acidic amino acid derivatives of 5-aminosalicylic acid. J Pharm Sci. 2001;90:1767-1775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Sinha VR, Kumria R. Polysaccharides in colon-specific drug delivery. Int J Pharm. 2001;224:19-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 504] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 15. | Watts PJ, Illum L. Colonic drug delivery. Drug Dev Ind Pharm. 1997;23:893-913 DOI : 10.3109/03639049709148695. |
| 16. | Chourasia MK, Jain SK. Pharmaceutical approaches to colon targeted drug delivery systems. J Pharm Pharm Sci. 2003;6:33-66. [PubMed] |
| 17. | Zou M, Okamoto H, Cheng G, Hao X, Sun J, Cui F, Danjo K. Synthesis and properties of polysaccharide prodrugs of 5-aminosalicylic acid as potential colon-specific delivery systems. Eur J Pharm Biopharm. 2005;59:155-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Chungi VS, Rekhi GS, Shargel L. A simple and rapid liquid chromatographic method for the determination of major metabolites of sulfasalazine in biological fluids. J Pharm Sci. 1989;78:235-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Hussain FN, Ajjan RA, Moustafa M, Anderson JC, Riley SA. Simple method for the determination of 5-aminosalicylic and N-acetyl-5-aminosalicylic acid in rectal tissue biopsies. J Chromatogr B Biomed Sci Appl. 1998;716:257-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |