INTRODUCTION
The prevalence of gastrointestinal (GI) disease is increasing in subjects aged 65 years and above. Among them, gastroesophageal reflux disease (GERD) and peptic ulcer are the most important and common GI disorders[1]. Peptic ulcer is one of the most common gastrointestinal diseases with 4-5% prevalence in the human society. Although it was speculated that food or stress was a main causative factor in the early 20th century, genetic and environmental factors are considered most relevant and it is generally accepted that their complicated correlation has a great role in the occurrence of peptic ulcer at present[2].
The number of patients with peptic ulcer is increasing as a result of complicated social activity and the continued advance of civilization. Whereas people over the middle age were predisposed to have peptic ulcer in the past, even teenaged group is also susceptible recently. Major etiologic factors of peptic ulcer include Helicobacter pylori (H pylori) infection, excessive use of drugs such as non steroidal anti-inflammatory drugs (NSAIDs), irregular eating habits, food containing causative materials such as linoleic acid, smoking, alcohol consumption, psychological, and physiological stress[3]. Chronic medication of NSAIDs often evokes dyspepsia, ulcer, hemorrhage, and perforation of upper gastrointestinal tract[4].
These side effects that arise from NSAIDs are augmented by the suppression of prostaglandin (PG) synthesis and neutrophil-mediated injury secondary to the production of inflammatory mediators such as tumor necrosis factor alpha (TNF-α) and leukotrienes (LTs)[5,6]. The risk factors responsible for the aggravation of gastrointestinal lesion by NSAIDs are believed to be age[7], gender[8], dyspepsia[9], clinical history ulcer and complicated ulcer disease[10] as well as heavy usage of alcohol and smoking[11] and H pylori infection[12].
Especially, NSAIDs and alcohol consumption increase the risk for major upper GI bleeding. Alcohol-induced disorders of the GI tract are very common[13]. Alcoholic gastritis subsequently leads to the impairment of the integrity of gastric mucosal barrier, contributing to acid reflux into the subluminal layers of the mucosa and submucosa. The underlying mechanism of mucosal lesions such as petechiae, hemorrhage, and erosion elicited by alcohol is similar to various NSAIDs[14].
In this connection, this study was carried out to investigate the effect of NSAIDs on the susceptibility of gastric lesion sensitized by serial administration of alcohol and preventative effect of DA-9601 (Stillen™), Artemisia asiatica extract, on the progression of gastric lesion and finally to reveal the underlying mechanism of action. DA-9601 is now on the market in South Korea and will be on sale in other Asian countries in the near future. It is reported to be effective to erosive gastritis[15] and to possess antioxidative and cytoprotective actions in models of gastric mucosal damage[16,17]. The active ingredient of DA-9601 is eupatilin, which is reported to prevent experimental gastric damage induced by a variety of noxious agents.
MATERIALS AND METHODS
Test materials and experimental animals
DA-9601 (Lot No. DA-9601-L-07) with 0.42% of active ingredient, eupatilin, was extracted from Artemisiae herba and supplied to this study after HPLC analysis in Dong-A pharmaceutical institute. (S)-6-methoxy-α-methyl-2-napthaleneacetic acid (Naproxen), 10% neutral formalin, sodium phosphate monobasic, sodium phosphate dibasic, ferric chloride (FeCl3), thiobarbituric acid (TBA), trichloroacetic acid (TCA), malondialdehyde (MDA), sulfosalicylic acid, DTNB, β-nicotineamide adenine dinucleotide phosphate reduced form (β-NADPH), glutathione reductase, hexadecyltrimethylammonium bromide (HTAB), O-dianisidine dihydrochloride, bovine serum albumin (BSA) were obtained from Sigma (USA). Prostaglandin (PG) E2 [125I] radioimmunoassay kit (NEN) was obtained by DuPont (USA) and p-nitrothiophenol was obtained from TCI (Japan). Absolute ethanol, ether, and hydrochloric acid were purchased from Duksan (Korea). DA-9601 was suspended in 5% hydroxypropylmethylcellulose (HPMC) dissolved in sterilized saline (v/v). Seven-week-old SPF male Sprague-Dawley rats were obtained from Charles River (Kanagawa, Japan) and acclimatized at least 1 wk under standard laboratory conditions [temperature: 23±2 °C, humidity range: 40-70%, ventilation: 15-20/h, luminous intensity: 150-300 lux, 12 h light/dark cycle (lighting: 7:00 to 19:00)]. The rats were given regular chow and UV-sterilized tap water ad libitum.
Preventative effect of DA-9601 on ethanol-induced acute experimental gastric lesion
Rats fasted for 24 h were given 50% ETOH orally twice a day (8:00 a.m. and 4:00 p.m.) for 5 d. After body weight changes and clinical signs such as diarrhea and mortality were carefully examined, DA-9601 and naproxen (NAP) were administered orally after the last administration of ethanol and fasting animals. Rats were assigned to one of six experimental groups; group I: normal age-matched control, group II: alcohol administration, group III: NAP 50 mg/kg without alcohol administration, group IV: alcohol+NAP, groupV: DA-9601 100 mg/kg pretreatment+NAP without alcohol admi-nistration, groupVI: alcohol administration+DA-9601 100 mg/kg+NAP. Four hours after NAP administration, rats were anesthetized with ether and total extirpation of the stomach was performed. Thirteen milliliters of 4 °C saline was injected into the lumen of isolated stomach and maintained for 30 min. Surface area (mm2) of gastric lesion was measured using optical microscope (Olympus ×10) following gastrotomy along with greater curvature. Small portion of tissue was fixed in 10% formalin solution and mucosal fluid collected from gastric mucosa stored at -74 °C refrigerator.
Homogenization of gastric mucosal fluid
Frozen mucosal fluid was thawed at room temperature and diluted with 1 mL Tris-HCl buffer (pH 7.4) per 100 mg and homogenized at 3 000 r/min for 10 min. Supernatant was stored at -20 °C.
Measurement of the level of malondialdehyde in gastric mucosal fluid
TBA method was applied to accomplish MDA measurement. Two millimole per liter of FeCl3 was added to 100 µL supernatant, which was agitated and incubated in 37 °C water bath for 30 min. Then 30% TCA, 0.75% TBA and 5 mol/L HCl were added as well and boiled for reaction in water bath for 15 min. Reacted mixture was cooled down to room temperature and centrifuged at 3 000 r/min for 10 min. Supernatant was assigned to measure absorbance at 550 nm thereby the amount of MDA (nmol/L/mg protein) was generated from standard curve of MDA.
Measurement of PGE2 in gastric mucosal fluid
The amount of PGE2 was measured with [125I] RIA kit. After sample and tracer were added to the assay buffer, they were mixed with anti-serum and incubated at 2-8 °C for 24 h. Precipitating reagent was added to the sample, agitated well and then incubated for 30 min at 2-8 °C. The amount of PGE2 (pg/mg protein) was measured with gamma counter using pellet obtained from centrifugation at 3 000 r/min for 30 min.
Measurement of the amount of glutathione in gastric mucosal fluid
Sample added to 4% sulfosalicylic acid was centrifuged at 3 000 r/min for the elimination of protein and reacted with 0.1 mol/L sodium phosphate buffer (pH 7.5) containing 6 mmol/L DTNB, 0.3 mmol/L NADPH, 50 unit glutathione reductase at room temperature. The amount of GSH (nmol/L/mg protein) was generated by the absorbance of p-nitrophenol measured at 412 nm using the supernatant from centrifugation at 3 000 r/min for 10 min.
Measurement of the amount of myeloperoxidase in gastric mucosal fluid
The amount of myeloperoxidase (mU/mg protein) was measured at gastric mucosa modifying the method of previous reports. After gastric mucosa was homogenized in 0.5% HTAB solution containing 50 mmol/L potassium phosphate (pH 6.5), homogenized samples were freezed and thawed thrice and homogenized. Supernatant was added to the reaction buffer containing O-dianisidine dihydrochloride and H2O2 and the amount of mye-loperoxidase was calculated from the absorbance of the reaction buffer at 460 nm at every 1 min.
Protein assay
Protein assay was performed by means of measuring absorbance at 535 nm compared using standard curve of BSA.
Statistical analysis
Data were expressed as the mean±SD and all statistical analyses were performed using SigmaStat® for Windows 2.0 software (Jandel Corporation, USA). Statistical significance was evaluated by one-way analysis of variance (ANOVA) followed by Bonferroni post hoc test or Dunnett’s test for a multiple comparison. P<0.05 were considered significant.
RESULTS
Preventative effect of DA-9601 on ethanol-induced acute experimental gastric lesion
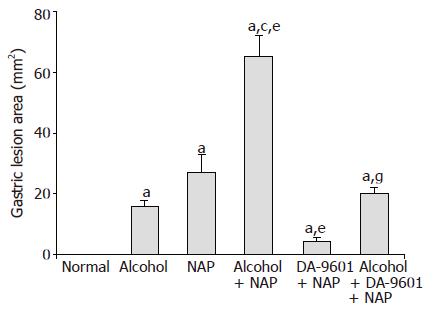
There was an apparent linear lesion on gastric mucosa of group II (alcohol) with area of gross lesion (mm2) of 16.0±1.8 (Figure 1). Group III (NAP) had a petechial lesion on gastric mucosa and area of gross lesion was 27.0±6.0. Group IV (alcohol+NAP) exhibited hemorrhage on gastric mucosa with a significantly different area of hemorrhagic lesion (65.3±6.7, P<0.05), which was more severe than groups II and III. Group V (DA-9601+NAP) significantly inhibited gross gastric lesion in comparison to group III (4.5±1.2, P<0.05). The area of gross gastric lesion of group VI (alcohol+DA-9601+NAP) was 20.0±2.1 which was significantly different from group IV (P<0.05).
Figure 1 The effect of DA-9601 on the area of gastric lesion induced by alcohol and NAP.
Data are expressed as mean±SD. aP<0.05 vs normal control, cP<0.05 vs alcohol group, eP<0.05 vs NAP, gP<0.05 vs alcohol + NAP.
MDA level in gastric mucosal fluid
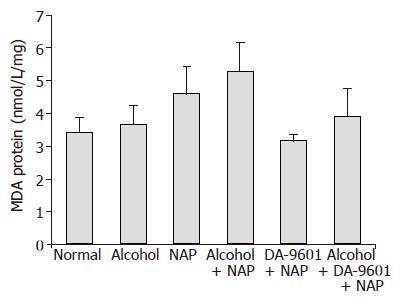
MDA levels (Figure 2) of groups I (normal control) and II (alcohol) indicating lipid peroxidation were 3.43±0.44 (nmol/L/mg protein) and 3.66±0.58 with no significant difference between them. It was found that groups III (NAP) and IV (alcohol+NAP) had 4.61±0.83 and 5.30±0.89, respectively. There was a tendency to increase MDA levels of groups III and IV without statistical significance. Group V (DA-9601+NAP) exhibited MDA level of 3.16±0.18 which was reduced by 31.5% compared with group III (NAP), but there was no significant difference between them. In case of group VI (alcohol+DA-9601+NAP), MDA level was 3.92±0.84 (nmol/L/mg protein) with 15.0% reduction compared to group IV, but no significance was found.
Figure 2 The effect of DA-9601 on MDA levels in gastric mucosal fluid.
There were no statistical significances among all groups.
Prostaglandin E2 level in gastric mucosal fluid
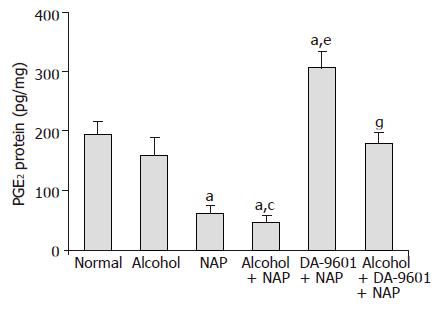
PGE2 level as a cytoprotection marker in group I (normal control) was determined to be 194.5±21.5 (pg/mg protein) (Figure 3), group II (alcohol) showed 159.5±30.2 which was reduced to the extent of 18.0% compared to group I (normal control) but did not produce significance. Group III (NAP) represented 62.0±13.8 indicating significant difference due to 68.1% reduction (P<0.05). PGE2 level of group IV (alcohol+NAP) was 47.1±11.9 which produced 70.5% reduction which was significantly different (P<0.05) from group II (alcohol) and 24.0% reduction without significance compared to group III (NAP). 307.2±27.3 was determined as the PGE2 level of group V (DA-9601+NAP) and it was 57.9% higher than normal (P<0.05) and 395.5% higher than group III (NAP) (P<0.05). Group VI (alcohol+DA-9601+NAP) showed 180.3±18.3 similar to group I (normal control) normal control but 282.8% increase with a significant difference (P<0.05) compared to group IV (alcohol+NAP).
Figure 3 The effect of DA-9601 on PGE2 levels in gastric mucosal fluid.
Data are expressed as mean±SD. aP<0.05 vs normal control, cP<0.05 vs alcohol group, eP<0.05 vs NAP, gP<0.05 vs alcohol + NAP.
Glutathione level
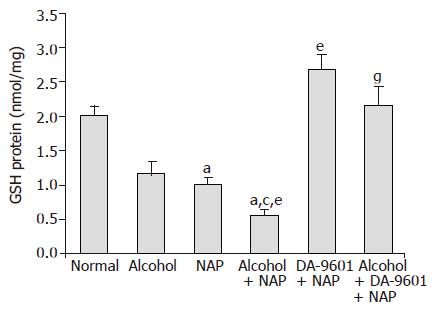
The levels of GSH in gastric mucosa (Figure 4) were 2.01±0.14 (nmol/L/mg protein) in group I (normal control) and 1.18±0.17 in group II (alcohol) and there was no significant difference between them. 1.01±0.21 of group III (NAP) produced 49.8% reduction with significant difference (P<0.05). GSH level of group IV (alcohol+NAP) was 0.55±0.10 which generated 53.4% and 45.5% reduction and was significantly different (P<0.05) from group II (alcohol) and group III (NAP), respectively. Group V (DA-9601+NAP) exhibited 2.68±0.22 with significant increase compared with group III, and that was even higher than group I (normal control). The level of GSH in group VI was 2.16±0.28 which was significantly higher than group IV (P<0.05).
Figure 4 The effect of DA-9601 on GSH levels in gastric mucosal fluid.
Data are expressed as mean±SD. aP<0.05 vs normal control, cP<0.05 vs alcohol group, eP<0.05 vs NAP, gP<0.05 vs alcohol + NAP.
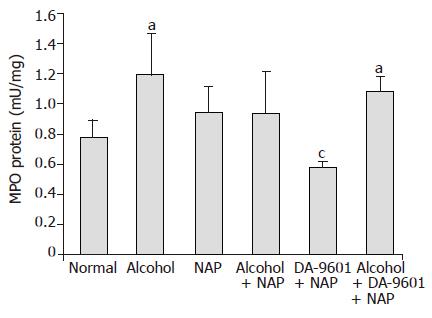
Myeloperoxidase level
The level of MPO in group I (normal control) was 0.78±0.11 (mU/protein) (Figure 5). Whereas group II (alcohol) exhibited significant increase of MPO (1.19±0.27) compared to normal (P<0.05), there was an increase of MPO in group III (NAP) without significance. 0.94±0.27 of group IV (alcohol+NAP) generated 21.1% reduction compared to group II, but was similar to group III. Group V (DA-9601+NAP) showed 0.58±0.04 which produced 25.6% reduction in comparison to group I (normal control) and was significantly decreased compared to group II (alcohol) and group III (NAP) (P<0.05). The level of MPO in group VI (alcohol+DA-9601+NAP) was 1.08±0.10 similarly to group II (alcohol) and group III (NAP).
Figure 5 The effect of DA-9601 on MPO levels in gastric mucosal fluid.
Data are expressed as mean±SD. aP<0.05 vs normal control, cP<0.05 vs NAP group.
Histopathological examination
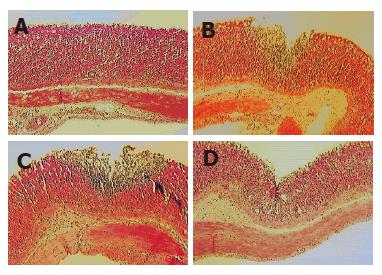
Histopathological changes are depicted in Figure 6. In group II (alcohol), there was a minimal inflammatory change in gastric mucosa; however, it was found that group III (NAP) had focal epithelial erosion and inflammatory cell infiltration in gastric mucosa and submucosa. Whereas group IV (alcohol+NAP) aggravated the gastric lesion by forming multifocal and diffuse epithelial erosion and severe inflammatory change in gastric mucosa and submucosa, DA-9601 treatment ameliorates the pathological changes of gastric mucosa and submucosa induced by NAP in alcohol pre-treated group (group VI).
Figure 6 Histopathological changes induced either by alcohol, NAP and alcohol + NAP administration and its inhibition by DA-9601.
A: Minimal inflammatory change in gastric mucosa in group II (alcohol); B: Focal epithelial erosion and inflammatory cell infiltration in gastric mucosa and submucosa of group III (NAP); C: Multifocal and diffuse epithelial erosion and severe inflammatory change in gastric mucosa and submucosa of Group IV (alcohol + NAP); D: DA-9601 treatment ameliorates the pathological changes of gastric mucosa and submucosa induced by NAP in alcohol pre-treated group VI (alcohol + DA-9601+NAP).
DISCUSSION
Considering the outcomes from this experiment, it was demonstrated that co-administration of alcohol and naproxen, one of the currently available NSAIDs, augmented the gastric mucosal lesion in comparison to the administration of alcohol or naproxen alone. Subsequently, treatment of DA-9601 that is being developed for targeting a new gastric mucoprotectant was shown to account for the preventative effects on gastric mucosa effectively against the lesion caused by alcohol and naproxen. Besides the effects on quantified gastric lesion, the level of MDA and MPO in DA-9601 treated group exhibited similar level to that in normal control and it was also revealed that DA-9601 contributed to intact maintenance of the gastric mucosa at the extent of normal control on histopathological examination.
The well-known etiologies for peptic ulcer can be categorized as follows: eating habits, environmental factors, stress by social life, drinking behavior, smoking, and drugs. Among these causative factors, drug-induced peptic ulcer is increasing dramatically with respect to the overuse of NSAIDs and increased susceptibility of gastric mucosa by single or co-administration of oral anti-coagulants (warfarin, phenindione, phenprocoumon, acenocoumarol, anisindione, diphenadione, etc.) and corticosteroid hormones (triamcinolone, dexamethasone, cortisone, hydrocortisone, prednisolone, prednisone)[18]. It is reported that alcohol is responsible for the hemorrhage of gastric mucosa and edema in submucosal muscle layer by means of direct irritation[19] and also leads to acute gastritis associated with microcircular stasis[20]. Once gastric mucosa is injured, thereby ischemia and decreased level of ATP in gastric mucosa and intervention of microcirculation occurs[21]. Gastric mucosal damage due to chronic ingestion of low concentration of alcohol is generally not discernible on gross examination; however, if gastric mucosa is exposed to an external mucosal irritant, gastric lesion becomes more extensive and consistent with increased susceptibility as well as persistent submucosal microcircular stasis[22]. It was demonstrated that DA-9601 100 mg/kg treatment exerted excellent preventative or therapeutic effects on gastric mucosal lesion with action of duration being effective for more than 2 h. It was also well documented that these effects were in accordance with the facilitation of the synthesis of PG by DA-9601.
Since aspirin was first synthesized in the year of 1899, a variety of NSAIDs has emerged so far and they are being used as antipyretic analgesics and anti-inflammatory drugs. Nowadays, the market size of NSAIDs is more than 60 billion/year, in addition, more extended indications are making the need for NSAIDs to increase[23]. Despite these facts, NSAIDs were reported to cause gastric lesion since 1930s and underlying mechanism was related to the fact that NSAIDs inhibit cyclooxygenase (COX) thereby block the synthesis of PG[24]. More specifically, NSAIDs elicit gastromucosal lesion not only by reducing the physiological role of PG, but also by directly inducing neutrophilic infiltration in gastric capillaries causing subsequent stasis and free radical formation by extravasated neutrophil[25]. Several methods are suggested to alleviate the adverse effects of NSAIDs until recently. First of all, aspirin is administered in the form of enteric-coated tablet or direct irritation to gastric mucosa is prevented by administration of prodrug[26]. Secondly, NSAIDs are administered to patients being chemically modified with nitric oxide that is known to act as a gastric mucosal protectant similarly to PG[27,28]. Lastly, NSAIDs with high sensitivity to COX II are designed[29,30]. Despite these efforts, 62.2% of patients treated with NSAIDs on a long-term basis complained of upper gastrointestinal clinical signs of which gastritis (38.5%), gastric ulcer (15.5%), duodenal ulcer (1.9%) were most prevalent; however, it appears that more patients suffered from these adverse effects due to complicated drug administration or eating habits. The underlying mechanisms that result in gastrointestinal symptoms can be classified as follows: firstly, it is generally accepted that NSAIDs inhibit COX responsible for the synthesis of PG. Secondly, NSAIDs have a direct effect on gastric mucosa and lastly it is also proposed that overproduction of LTs, especially for LTB4, by 5-lipoxygenase (5-LO) resulting from compensation for the inhibition of COX is a causative factor[31,32]. LTs are known to be mediators that induce inflammatory process and take part in the formation and infiltration of neutrophils[33]. The most commonly prescribed formula considering NSAIDs treatment is the co-administration of extrinsic or intrinsic PG. Whereas Misoprostol, one of the extrinsic PG synthetics, has the advantage in the aspect of potency, and it exhibits adverse effect such as diarrhea. The intrinsic PG derivative, ornoprostil, is known to be not effective in the patients with gastric ulcer or gastritis because of the fact that NSAIDs inhibit COX[34]. It was revealed that DA-9601 increased the level of PGE2 and GSH dose-dependently in normal state as well as alcohol or ammonia-induced gastric lesion. Moreover, DA-9601 elevated the level of PGE2 protecting gastric mucosa without affecting the efficacy of NSAIDs, when DA-9601 was co-administered with NSAIDs in arthritis-induced rats. Oxygen free radical and lipid peroxidation are regarded as the crucial etiologies leading to gastric mucosal lesion by oxidative stress[35]. Once oxygen free radical triggers and maintains ischemic status in gastric mucosa, hydroxyl radical generated from superoxide anion results in gastric lesion by virtue of decreasing the velocity of blood flow subsequently[36].
In this present study, we measured not only the level of MDA and MPO in order to quantify the materials contributing to gastric mucosal lesion but also the level of PGE2 and GSH that are produced as protective markers. As a whole, it was demonstrated that the level of MDA and MPO were decreased similarly to the level of normal control, whereas PGE2 and GSH were increased in DA-9601 treated group. In the previous report, DA-9601 treatment alone had the ability to facilitate the production of mucosal PGE2[17]. From the findings of this study, DA-9601 treatment was proven to protect effectively against the NSAIDs-induced lesion of gastric mucosa which was more susceptible to NSAIDs by the administration of alcohol in rats. On closer inspection of results, the underlying mechanism of the action of DA-9601 can be centered on the cytoprotective effect by normalizing the production of PG, additional improvement of gastric mucosal function by elevating GSH content, alleviation of inflammation by decreasing the level of MDA and MPO. Bearing all these in mind, it is worth making a conclusion that DA-9601 treatment has a protective effect on gastric mucosa of patients who are accustomed to drinking alcohol regularly and will be of great value in human clinical study.