Published online Dec 14, 2005. doi: 10.3748/wjg.v11.i46.7290
Revised: January 23, 2005
Accepted: January 26, 2005
Published online: December 14, 2005
AIM: To examine an increased risk of esophageal adenocarcinoma is restricted to patients who develop Barrett’s esophagus or whether esophagitis per se is a risk factor for adenocarcinoma.
METHODS: A population-based cohort of patients with histological evidence of esophagitis without Barrett’s esophagus was constructed using electronic pathology reports relating to all esophageal biopsies in Northern Ireland between 1993 and 1996. Person-years of follow-up and incident cases of esophageal cancer were calculated by linking the cohort to death files and the Northern Ireland Cancer Registry records. Standardized incidence ratios (SIR) were calculated for esophageal cancers (adenocarcinoma, squamous cell carcinoma (SCC), and histologically unspecified cancers).
RESULTS: A total of 2 013 patients in the cohort provided 13 559 patient-years of follow-up (mean follow-up 6.7 years). None of the patients developed adenocarcinoma. Three patients developed SCC, and six developed histologically unspecified cancers. The SIR for all esophageal cancers and for SCC were 2.73 (95%CI 1.25-5.19) and 2.93 (95%CI 0.61-8.59), respectively. In a sensitivity analysis in which all unspecified esophageal cancers were treated as adenocarcinomas, the SIR for adenocarcinoma was 2.64 (0.97-5.75).
CONCLUSION: The risk of adenocarcinoma is not elevated in patients with histological evidence of esophagitis without Barrett’s esophagus; however, these patients may have a moderately increased risk of SCC. Further studies are required to confirm these findings, which suggest that Barrett’s esophagus, not esophagitis, is the key precursor lesion in the development of adenocarcinoma.
- Citation: Murphy SJ, Anderson LA, Johnston BT, Fitzpatrick DA, Watson PR, Monaghan P, Murray LJ. Have patients with esophagitis got an increased risk of adenocarcinoma? Results from a population-based study. World J Gastroenterol 2005; 11(46): 7290-7295
- URL: https://www.wjgnet.com/1007-9327/full/v11/i46/7290.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i46.7290
The incidence of esophageal adenocarcinoma (OAC) is increasing at a rate faster than any other cancer in the Western world[1]. The most important risk factor for its development is Barrett’s esophagus (BO), a metaplastic condition in which the native squamous epithelium of the esophagus is replaced by columnar epithelium, in response to chronic gastro-esophageal reflux[2]. OAC appears to result from a sequence of changes from esophagitis to non-dysplastic BO, to dysplastic BO, and finally to adenocarcinoma[3,4]. It is generally accepted that most, if not allpatients who develop OAC, pass through this sequence. It is on this basis that endoscopic surveillance of BO is recommended, in an attempt to reduce mortality from OAC[5]. However, some aspects of the relationship between BO and OAC remain unclear. While BO is the only known precursor to this tumor, there is a huge variation in the proportion of cases of OAC in which it is detectable, ranging from 23% to 100% of cases in different studies[6-12]. The difficulty in identifying BO in patients with OAC is thought to result from tumor overgrowth of the Barrett’s segment. However, in a large prospective case-control study in Sweden, Lagergren et al[13] found that in patients with OAC, only 62% had evidence of BO identified by biopsy, despite extensive sampling by a rigorous protocol. They also found that symptoms of gastro-esophageal reflux per se were strongly associated with adenocarcinoma, and that the presence or absence of BO in cancer cases had no effect on the strength of this association. These findings have led to the speculation that gastro-esophageal reflux, rather than BO, is the crucial factor in the development of OAC. If that is the case, then esophagitis may be an important risk factor for its development. To our knowledge, there are no studies assessing the risk of OAC in patients with biopsy-proven esophagitis. We undertook a study to examine the incidence of OAC in a population-based cohort of patients who had histological evidence of esophagitis without BO.
This study is a follow-up study of a population-based cohort of patients with esophagitis. The cohort comprised every adult within Northern Ireland (NI), population 1.7 million, with histological evidence of inflammation of the esophagus that was not due to infection or radiation. The cohort was constructed by examining pathological reports relating to all esophageal biopsies undertaken within all the hospitals in NI between January 1993 and December 1996. Data were available for biopsies taken between 1993 and 1999, but only the first 4 years were used in order to maximize the period of follow-up. These reports were made available to the NI Cancer Registry (NICR) in an electronic format during the construction of the NI Barrett’s Register[14]. The reports contained information on the nature and site of the submitted biopsy specimen, the clinical summary recorded by the endoscopist on the request form, the full text of the pathologist’s report on the specimen, and the pathologist’s diagnosis. The clinical summaries contained information relating to the site at which the biopsies were taken. All pathological reports in which the summary stated that the biopsies were taken from the esophagus, including the esophago-gastric junction (OGJ), were examined. A list of SNOMED (Systematized nomenclature of medicine)[15] codes indicating inflammation in the esophagus was compiled and biopsies were included in the study, if any of these codes appeared on the biopsy report (Table 1). The report which included more than one diagnostic code was considered if at least one of the codes indicated inflammation .
| Code | Diagnostic term | Number of patients |
| M40000 | Inflammation | 777 |
| MZ0005 | Non-specific inflammation | 9 |
| M40005 | Active inflammation | 88 |
| M41000 | Acute inflammation | 31 |
| M42100 | Active chronic inflammation | 62 |
| M43000 | Chronic inflammation | 279 |
| M76820 | Inflammatory polyp | 2 |
| M36500 | Edema | 2 |
| M36100 | Congestion | 52 |
| M45020 | Granulation tissue | 11 |
| M14110 | Erosion | 6 |
| M38000 | Ulcer/ulceration | 570 |
| MY0102 | Reflux esophagitis | 125 |
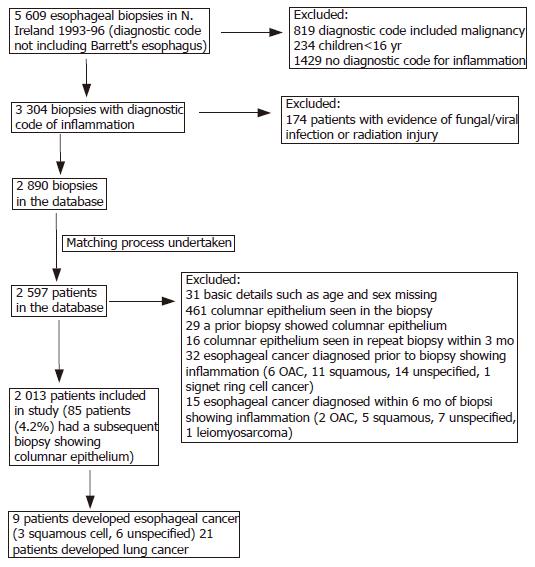
Biopsy reports were excluded, if patients were less than 16 years of age, or if the diagnostic codes included malignancy, fungal or viral infection, or radiation injury. The bodies of the reports were then examined and any reports in which the pathologist recorded the presence of columnar epithelium were excluded.
A substantial number of patients in the cohort had esophageal biopsies taken on more than one occasion. Individual patients were identified within the dataset by matching on surname, forename(s), and date of birth (and hospital numbers when they were available). The date of the earliest biopsy showing the inflammation was taken as the date of entry into the cohort.
Some patients had an esophageal biopsy that contained columnar epithelium prior to their first biopsy showing inflammation (NB data only available from January 1993). These patients were excluded from the cohort. Other patients had an esophageal biopsy subsequent to their first biopsy showing inflammation that showed columnar epithelium. These patients were not excluded, unless the biopsy showing columnar epithelium occurred within 3 mo of the initial biopsy. These steps were taken to exclude prevalent cases of BO but to include incident cases in the cohort, the rationale being that if esophagitis leads to malignancy through the development of BO, excluding incident BO would potentially miss these patients.
Members of the cohort were followed up for deaths and incident esophageal cancer OAC, squamous cell carcinoma (SCC), and histologically unspecified cancers and lung cancer till the end of December 2002. Lung cancer incidence within the cohort was determined as a proxy for smoking exposure. Deaths among the cohort were identified by matching with the death files from the Registrar General’s Office (NI) using the patient’s surname, forename(s), and date of birth. These files contained information on all the deaths that occurred within NI.
Incident cases of esophageal cancer with a date of diagnosis at least 6 mo after the date of entry into the cohort were identified by matching the cohort with the NICR database (using the patient’s surname, forename(s), date of birth, and hospital numbers where available). This population-based cancer registry had collected data on all cancers occurring in NI residents, since the beginning of 1993. When assessing lung cancer incidence, a threshold of 3 mo after the date of entry into the cohort was used.
Person-years of follow-up were calculated for each member of the cohort with censoring either on the date of diagnosis of the esophageal or lung cancer, on the date of death, or on 31st December 2002. Incidences of OAC, SCC, histologically unspecified esophageal cancers, and lung cancer were calculated as the number of events divided by the person-years of follow-up. These incidences were expressed as events per hundred person-years of follow-up, which is equivalent to percentage incidence per year. The standardized incidence ratio (SIR) for these cancers was calculated by comparing the observed number of cancers in the cohort with the expected number, and by applying the relevant age-specific cancer incidence rates in the NI population to the cohort. Exact confidence intervals (CI) of rates and ratios were estimated using the Poisson distribution. Sensitivity analyses were performed in which histologically unspecified esophageal cancers were reclassified as OACs and then as SCCs and the corresponding SIRs were recalculated. This was done to illustrate the most extreme case scenarios, by assuming that all unspecified cancers were one or the other histological type of cancer. These analyses are secondary analyses of data collected during the construction of the NI Barrett’s Register. Establishment of this Register received ethical approval from the Research Ethics Committee of the Queen’s University, Belfast.
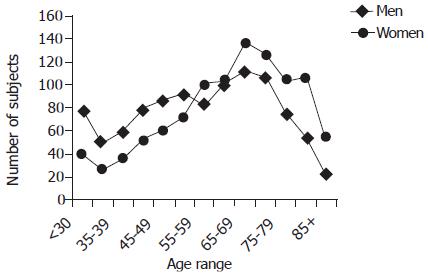
Figure 1 shows how the cohort was constructed and the effect of exclusion criteria. There were 2 013 patients in the cohort, comprising 996 (49.5%) men and 1 017 (50.5%) women. The mean age was 59.8 years, range 16.2-95.6 years. Men were substantially younger than women (56.6±17.0 vs 63.0±16.2 years). The age and sex distributions of patients are shown in Figure 2. In 90 (4.5%) patients, the biopsies had been taken at the OGJ.
Members of the cohort were followed up for a mean of 6.7 years for a total of 13 559 person-years of follow-up. Nine patients developed esophageal cancer (4 men and 5 women), a mean of 3.1 years (range, 1.0-6.8 years) after the initial biopsy showing esophagitis. Mean age at diagnosis was 75.1 years (range, 60.8-86.3 years). None of the incident esophageal cancers were adenocarcinomas; three were SCCs and six were histologically unspecified. Two of the three patients who developed SCC were women. Histological confirmation of cancer was not possible in five of the six unspecified tumors, as biopsies were not taken. These diagnoses were based on the clinical opinion and in one case the registered causes of death did not include esophageal cancer. The pathological diagnosis in the single unspecified cancer case in whom biopsies had been taken was recorded as anaplastic carcinoma but in the body of the report the pathologist stated that it was ‘probably squamous in type’.
The incidences and SIRs of esophageal cancers are shown in Table 2. The SIR (95%CI) for all esophageal cancers in the cohort was 2.73 (1.25-5.19), with a higher risk in women than men. The SIR (95%CI) for SCC was also raised at 2.93 (0.61-8.59) but this did not reach conventional statistical significance. The SIR for adenocarcinoma was 0 with an upper 97.5%CI of 2.73. None of the esophageal cancers occurred in patients whose biopsies were taken at the OGJ and exclusion of these patients had little effect on observed SIRs (data not shown). In the sensitivity analyses in which histologically unspecified cancers were reclassified as OACs and then as SCCs, the SIRs (95%CI) for OAC and SCC were 2.64 (0.97-5.75) and 4.64 (2.12-8.81), respectively. Dysplasia was noted in 20 (1%) patients; none of these patients developed esophageal cancer.
| Number ofcases | Incidence per 100 person-years of follow-up (95%CI) | Expected numberof cases | Standardized incidenceratio (95%CI) | ||
| OAC | All patients | 0 | - | 1.35 | 0 ( -, 2.73)1 |
| All esophageal cancers | All patients | 9 | 0.07 (0.04, 0.14) | 3.29 | 2.73 (1.25-5.19) |
| Men | 4 | 0.06 (0.02, 0.16) | 1.8 | 2.22 (0.61-5.69) | |
| Women | 5 | 0.07 (0.02, 0.17) | 1.43 | 3.49 (1.14-8.16) | |
| Esophageal | All patients | 3 | 0.02 (0.01, 0.07) | 1.02 | 2.93 (0.61-8.59) |
| SCC | |||||
| Men | 1 | 0.02 (0, 0.10) | 0.44 | 2.28 (0.06-12.6) | |
| Women | 2 | 0.03 (0, 0.12) | 0.59 | 3.40 (0.41-12.2) | |
| Lung cancer | All patients | 21 | 0.16 (0.10, 0.24) | 19.71 | 1.07 (0.66-1.63) |
| Men | 16 | 0.25 (0.14, 0.40) | 11.36 | 1.41 (0.81-2.29) | |
| Women | 5 | 0.07 (0.02, 0.17) | 7.63 | 0.66 (0.21-1.53) | |
| Sensitivity analyses | |||||
| Reclassified OAC2 | All patients | 6 | 0.04 (0.01, 0.13) | 2.27 | 2.64 (0.97-5.75) |
| Men | 3 | 0.05 (0.02, 0.11) | 1.36 | 2.20 (0.45-6.45) | |
| Women | 3 | 0.04 (0.01, 0.13) | 0.85 | 3.54 (0.73-10.31) | |
| Reclassified esophageal SCC3 | All patients | 9 | 0.07 (0.02, 0.17) | 1.94 | 4.64 (2.12-8.81) |
| Men | 4 | 0.06 (0.02, 0.16) | 0.91 | 4.39 (1.20-11.25) | |
| Women | 5 | 0.07 (0.02, 0.17) | 1.02 | 4.92 (1.59-11.44) | |
Eighty-five (4.2%) patients in the cohort had a subsequent esophageal biopsy (after a minimum of 3 mo and before December 1999) showing columnar epithelium. None of the patients developed esophageal cancers in this group.
Twenty-one patients developed lung cancer, including 16 men and 5 women. Mean age at diagnosis of lung cancer was 71.3 years (range, 32.7-80.6 years). Overall, the SIR for lung cancer was not raised although a modest increase in risk was seen in men, which was not statistically significant (SIR 1.41, 95%CI 0.81-2.29).
This is the first population-based study to assess the risk of esophageal cancer in patients with histologically proven esophagitis that is not complicated by BO. We found that none of the 2 013 patients with esophagitis developed OAC after an average follow-up of 6.7 years. However, some of the six histologically unspecified tumors that occurred within the cohort might be adenocarcinomas.
This study has got many advantages. All patients had histologically confirmed esophagitis, and every patient in whom this diagnosis was made in NI between 1993 and 1996 was included in the study. The population-based nature of this study avoids selection biases that may operate when only patients attending specific centers are investigated. Also, patients who showed evidence of BO were excluded from the cohort, which allowed us to examine the risk of esophageal cancer in esophagitis uncomplicated by BO. Finally, follow-up of patients is likely to be near-completion for two reasons: firstly, population-based cancer and death registers were used to determine deaths and cancer incidence among the cohort; and secondly, emigration from NI is uncommon, averaging 1.1% of the population per year in the period 1991-2003[16].
This study also has limitations. A diagnosis of ‘reflux esophagitis’ was made in only a small proportion of cases because scant clinical details were provided on the pathology request forms. However, reflux of gastric contents was the most likely cause of esophagitis that was not due to infection or radiation. Also, patients with esophagitis were not routinely biopsied, so patients included in this cohort may represent a subset of patients with esophagitis who had suspicious features at endoscopy. Risk of esophageal cancer may, therefore, be exaggerated in this cohort but exclusion of prevalent cancers and cancers occurring within the first 6 mo reduces the effect of this problem. Accurate histological classification of the incident esophageal tumors occurring in the cohort was not available for 60% of tumors. Some of the unclassified tumors may be adenocarcinomas rendering the absolute incidence of OAC, an underestimate. However, classification of esophageal cancer incidence in the general population is subject to the same limitations, meaning that the SIRs presented for all cancers and for cancer subgroups should be valid comparisons between the cohort and the general population. Furthermore, we performed sensitivity analyses to estimate the maximum incidence of OAC and SCC, by assuming that all the histologically unspecified cancers were actually OACs or SCCs, respectively. Lastly, the modest size of the cohort and the period of follow-up have resulted in small numbers of incident cancers, which may render some of the estimates of cancer risk unstable. Another effect of the relatively short period of follow-up is that a late increase in the risk of OAC associated with esophagitis cannot be discounted.
When all histologically unspecified cancers were treated as OACs, giving a maximal possible risk of OAC, the risk in the cohort was approximately 2.5 times than that of the general population. This is undoubtedly an overestimate but the extent cannot be ascertained. We have previously demonstrated a 17-fold increase in risk of OAC in a cohort of patients with BO (histologically confirmed specialized intestinal metaplasia) drawn from the same population and followed up in the same manner as in this study[14]. These data suggest that, unlike BO, esophagitis without BO is not an important risk factor for OAC.
The literatures regarding the risk of adenocarcinoma in esophagitis are conflicting. Previous case-control studies have shown that esophagitis or esophageal ulcer is associated with a two- to fivefold increased risk of OAC[17-19]. However, the diagnosis of esophagitis was obtained from case-note review or questionnaire and was not histologically confirmed. Also, it was not possible to identify and exclude patients with BO in these studies. Ye et al[20] reported a sixfold increase in the incidence of OAC in a large population-based cohort study of patients with a hospital diagnosis of gastro-esophageal reflux disease (GORD), but patients with BO were not excluded. A recent study utilizing data from the United Kingdom General Practice Research Database estimated that patients with esophagitis had a 4.5-fold increased risk of OAC compared to the normal population, whereas the risk in BO patients was increased 30-fold[21]. Moreover, following long-term follow-up of patients with GORD, Spechler et al[22] found that patients with BO at baseline developed OAC at an annual rate of 0.4%, whereas the rate in patients without BO was only 0.07%. These data from patients with clinical diagnoses of esophagitis and BO appear to be in agreement with our findings from patients with histological confirmation of their diagnoses.
Only 4.5% of patients in our cohort had a subsequent esophageal biopsy showing columnar epithelium, suggesting that progression from esophagitis to BO may be uncommon. However, it must be borne in mind that there was no systematic recall of patients for endoscopy, so the incidence of BO in the cohort may be underestimated. Also, a systematic biopsy protocol was not employed, so columnar epithelium may have been missed in some patients, either in their initial biopsy or subsequent biopsies.
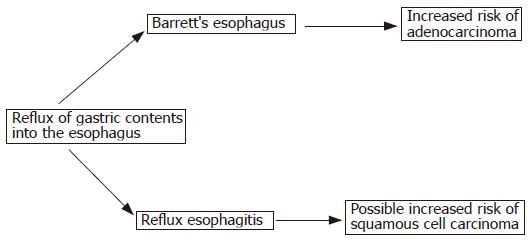
The low incidence of OAC in our cohort suggests that progression along a path from esophagitis to BO and to OAC may not be the normal sequence of events. Rather, an individual’s response to reflux of gastric contents may result in either esophagitis or BO, with a future risk of OAC being confined to those who develop BO. Cameron and Arora[23] carried out a detailed study in which they mapped areas of damage seen at endoscopy in patients with esophagitis and in patients with BO. Their findings suggest that BO is unlikely to develop directly from areas of esophagitis, adding plausibility to the proposed pathway in Figure 3.
The incidence of SCC within the cohort was almost three times than that of the general population. There is limited evidence in the literature supporting a link between esophagitis and SCC. Munoz et al[24] showed a very high prevalence of esophagitis (84%) in people living in the Linxian region of China, where there is a very high incidence of esophageal SCC. However, the authors stated that this esophagitis affected the middle and lower thirds of the esophagus as opposed to the distal esophagus; also, it was not accompanied by erosions or ulcers, features characteristic of esophagitis secondary to gastro-esophageal reflux. These patients are very different from the cohort that we had examined, particularly in terms of lifestyle and nutritional status and gastro-esophageal reflux may not be important in the etiology of esophagitis in China. Ribet et al[25] found evidence of an association between reflux symptoms and SCC in the patients who underwent resection of esophageal cancers, but this was a small study involving a highly selected group. Ye et al[20] found a moderately raised risk of SCC in a Swedish cohort of hospital patients with GERD. On the other hand, in a large Swedish case-control study, Lagergren et al[13] found no association between symptoms of reflux and risk of SCC of the esophagus.
Our finding of increased SCC risk in patients with esophagitis could result from over-representation of smokers within the cohort, since smoking is associated with reflux symptoms[26,27]. However, this was unlikely because the risk of lung cancer was not raised in the cohort. It may also result from inclusion of patients with suspicious lesions seen at endoscopy, which might include dysplastic squamous epithelium; however, less than 1% of patients had evidence of dysplasia, and none of these developed SCC. Further research is required to examine the association we have seen between esophagitis and SCC.
In summary, our study shows that esophagitis that is not complicated by BO is not associated with an increased risk of OAC. This finding supports the view that BO, not esophagitis, is the key precursor of OAC. Further studies are required to see if these findings can be replicated. Determination of the relative importance of esophagitis in esophageal cancer causation is important because this has implications for the management of patients with gastro-esophageal reflux and for the prevention of OAC.
The authors thank Dr. Dermot Hughes, Consultant Pathologist at Altnagelvin Area Hospital, for advice relating to SNOMED codes used in the study. Thanks are also due to Mr. Colin Fox, Mr. Richard Middleton, and the Tumor Verification Officers of the NICR for their assistance with the processing of pathological records. We are also grateful to Dr. Anna Gavin, Director of the NICR and to the administrative, medical and pathology staff of local Health Care Trusts and the staff of the Directorate of Information Services DHSSPS (NI) for assistance in the construction of the NI Barrett’s Register.
Science Editor Kumar M and Guo SY Language Editor Elsevier HK
| 1. | Devesa SS, Blot WJ, Fraumeni JF. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83:2049-2053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 2. | Spechler SJ, Goyal RK. Barrett's esophagus. N Engl J Med. 1986;315:362-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 582] [Cited by in RCA: 507] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 3. | Reid BJ, Sanchez CA, Blount PL, Levine DS. Barrett's esophagus: cell cycle abnormalities in advancing stages of neoplastic progression. Gastroenterology. 1993;105:119-129. [PubMed] |
| 4. | Altorki NK, Oliveria S, Schrump DS. Epidemiology and molecular biology of Barrett's adenocarcinoma. Semin Surg Oncol. 1994;13:270-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Sampliner RE. Practice guidelines on the diagnosis, surveillance, and therapy of Barrett's esophagus. The Practice Parameters Committee of the American College of Gastroenterology. Am J Gastroenterol. 1998;93:1028-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 390] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 6. | Haggitt RC, Tryzelaar J, Ellis FH, Colcher H. Adenocarcinoma complicating columnar epithelium-lined (Barrett's) esophagus. Am J Clin Pathol. 1978;70:1-5. [PubMed] |
| 7. | Hamilton SR, Smith RR, Cameron JL. Prevalence and characteristics of Barrett esophagus in patients with adenocarcinoma of the esophagus or esophagogastric junction. Hum Pathol. 1988;19:942-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 173] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 8. | Duhaylongsod FG, Wolfe WG. Barrett's esophagus and adenocarcinoma of the esophagus and gastroesophageal junction. J Thorac Cardiovasc Surg. 1991;102:36-41; discussion 41-42. [PubMed] |
| 9. | Streitz JM, Ellis FH, Gibb SP, Balogh K, Watkins E. Adenocarcinoma in Barrett's esophagus. A clinicopathologic study of 65 cases. Ann Surg. 1991;213:122-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 84] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Li H, Walsh TN, Hennessy TP. Carcinoma arising in Barrett's esophagus. Surg Gynecol Obstet. 1992;175:167-172. [PubMed] |
| 11. | Clark GW, Smyrk TC, Burdiles P, Hoeft SF, Peters JH, Kiyabu M, Hinder RA, Bremner CG, DeMeester TR. Is Barrett's metaplasia the source of adenocarcinomas of the cardia? Arch Surg. 1994;129:609-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 191] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 12. | Cameron AJ, Lomboy CT, Pera M, Carpenter HA. Adenocarcinoma of the esophagogastric junction and Barrett's esophagus. Gastroenterology. 1995;109:1541-1546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 280] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 13. | Lagergren J, Bergström R, Lindgren A, Nyrén O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med. 1999;340:825-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2115] [Cited by in RCA: 2026] [Article Influence: 77.9] [Reference Citation Analysis (0)] |
| 14. | Murray L, Watson P, Johnston B, Sloan J, Mainie IM, Gavin A. Risk of adenocarcinoma in Barrett's oesophagus: population based study. BMJ. 2003;327:534-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 92] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Spackman KA, Campbell KE, Côté RA. SNOMED RT: a reference terminology for health care. Proc AMIA Annu Fall Symp. 1997;640-644. [PubMed] |
| 16. | Northern Ireland Migration Flows 1991-2003. Available from: http://www.nisra.gov.uk/statistics/financeandpersonnel/dmb/datavault.ht. |
| 17. | Chow WH, Finkle WD, McLaughlin JK, Frankl H, Ziel HK, Fraumeni JF. The relation of gastroesophageal reflux disease and its treatment to adenocarcinomas of the esophagus and gastric cardia. JAMA. 1995;274:474-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 150] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Farrow DC, Vaughan TL, Sweeney C, Gammon MD, Chow WH, Risch HA, Stanford JL, Hansten PD, Mayne ST, Schoenberg JB. Gastroesophageal reflux disease, use of H2 receptor antagonists, and risk of esophageal and gastric cancer. Cancer Causes Control. 2000;11:231-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 141] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 19. | Wu AH, Tseng CC, Bernstein L. Hiatal hernia, reflux symptoms, body size, and risk of esophageal and gastric adenocarcinoma. Cancer. 2003;98:940-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 144] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 20. | Ye W, Chow WH, Lagergren J, Yin L, Nyrén O. Risk of adenocarcinomas of the esophagus and gastric cardia in patients with gastroesophageal reflux diseases and after antireflux surgery. Gastroenterology. 2001;121:1286-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 174] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 21. | Solaymani-Dodaran M, Logan RF, West J, Card T, Coupland C. Risk of oesophageal cancer in Barrett's oesophagus and gastro-oesophageal reflux. Gut. 2004;53:1070-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 207] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 22. | Spechler SJ, Lee E, Ahnen D, Goyal RK, Hirano I, Ramirez F, Raufman JP, Sampliner R, Schnell T, Sontag S. Long-term outcome of medical and surgical therapies for gastroesophageal reflux disease: follow-up of a randomized controlled trial. JAMA. 2001;285:2331-2338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 660] [Cited by in RCA: 583] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 23. | Cameron AJ, Arora AS. Barrett's esophagus and reflux esophagitis: is there a missing link? Am J Gastroenterol. 2002;97:273-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Muñoz N, Crespi M, Grassi A, Qing WG, Qiong S, Cai LZ. Precursor lesions of oesophageal cancer in high-risk populations in Iran and China. Lancet. 1982;1:876-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 88] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Ribet ME, Mensier EA. Reflux esophagitis and carcinoma. Surg Gynecol Obstet. 1992;175:121-125. [PubMed] |
| 26. | Murray L, Johnston B, Lane A, Harvey I, Donovan J, Nair P, Harvey R. Relationship between body mass and gastro-oesophageal reflux symptoms: The Bristol Helicobacter Project. Int J Epidemiol. 2003;32:645-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 164] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 27. | Locke GR, Talley NJ, Fett SL, Zinsmeister AR, Melton LJ. Risk factors associated with symptoms of gastroesophageal reflux. Am J Med. 1999;106:642-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 339] [Article Influence: 13.0] [Reference Citation Analysis (0)] |