Published online Nov 21, 2005. doi: 10.3748/wjg.v11.i43.6867
Revised: April 26, 2005
Accepted: April 30, 2005
Published online: November 21, 2005
AIM: To evaluate the effect of MSX2 on gemcitabine-induced caspase-3 activation in pancreatic cancer cell line Panc-1.
METHODS: Using V5-tagged MSX2 expression vector, stable transfectant of MSX2 was generated from Panc-1 cells (Px14 cells). Cell viability under gemcitabine administration was determined by MTT assay relative to control cell line (empty-vector transfected Panc-1 cells; P-3EV cells). Hoechst staining was used for the detection of apoptotic cell. Activation of caspase-3 was assessed using Western blotting analysis and direct measurement of caspase-3 specific activities.
RESULTS: MSX2 overexpression in Panc-1 cells resulted in decreased gemcitabine-induced caspase-3 activation and increased cell viability under gemcitabine treatment in Px14 cells.
CONCLUSION: MSX2 exerts repressive effects on gemcitabine-induced apoptotic pathway. This novel apoptosis-regulating function of MSX2 may provide a new therapeutic target for pancreatic cancer.
- Citation: Hamada S, Satoh K, Kimura K, Kanno A, Masamune A, Shimosegawa T. MSX2 overexpression inhibits gemcitabine-induced caspase-3 activity in pancreatic cancer cells. World J Gastroenterol 2005; 11(43): 6867-6870
- URL: https://www.wjgnet.com/1007-9327/full/v11/i43/6867.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i43.6867
Pancreatic cancer is known as a cancer with poor prognosis. Surgical resection of pancreatic cancer is available only in 15-20% of all patients[1], while medical approaches, such as chemotherapy or radiation, have no cure. The resistance of pancreatic cancer to chemotherapeutic agents is one of the serious problems in clinical situation. Gemcitabine (2’,2’-difluoro-2’-deoxycytidine) is a widely used chemotherapeutic agent for unresectable pancreatic cancer treatment, and its administration triggers apoptosis in pancreatic cancer cell line[2]. On the other hand, previous report showed that acquired gemcitabine resistance was accompanied by altered expression of apoptosis regulatory genes[3]. The mechanisms how cancer cells evade apoptotic signals are beginning to come to light, but its upstream regulators are not fully understood as yet.
We have previously demonstrated that overexpression of homeobox gene MSX2 accelerated proliferation of pancreatic cancer cell lines, combined with epithelial-mesenchymal transition (EMT)-like phenotypic changes in vitro (Satoh et al, submitted). In addition, forced expression of MSX2 increased anchorage-independent growth of pancreatic cancer cells, indicating enhanced aggressive biological behavior. Recent studies have demonstrated that MSX2 is a downstream target of the Wnt signaling pathway in several cancer cell lines[4,5]. The upregulation of Wnt signaling is reported in various cancer cell lines, and its target genes are closely related to cancer cell growth and survival[6-9]. At this point of view, we hypothesized that MSX2 might affect apoptotic signaling pathway, which leads to the chemoresistance of pancreatic cancer cells.
In this study, we generated MSX2-overexpressing pancreatic cancer cell line Px14, and this cell line revealed resistance to the gemcitabine treatment. When cells were treated with gemcitabine, the caspase-3 activation was significantly upregulated in empty vector-transfected control cells, but not in Px14 cells compared to basal control cells. Taken together, MSX2 might act as a negative regulator of apoptosis in this cell line. This new upstream regulator of apoptotic signaling pathway may provide a novel therapeutic target of chemotherapy-resistant pancreatic cancer.
Panc-1, a human pancreatic cancer cell line, was maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 100 mL/L fetal bovine serum (FBS), and incubated at 37 °C in a humidified atmosphere containing 50 mL/L CO2 in air.
Gemcitabine (Gemzar; Eli Lilly Co., Indianapolis, IN, USA) was dissolved in phosphate buffered saline (PBS) at various concentrations of 0.1, 1, 10, 100 μg/mL and 1 mg/mL as stock solutions. In cell culture, vehicle and these stock solutions were used in 1:100 dilutions.
Expression vector, pcDNA3.1-MSX2V5, was generated as described previously (Satoh et al submitted). Panc-1 cells were plated in six-well plates and cultured until reaching a subconfluent state. Cells were transfected with 1 μg of pcDNA3.1-V5His (empty vector) or pcDNA3.1-MSX2V5 using FuGENE6 transfection reagent (Roche Diagnostics, Basel, Switzerland) in normal growth medium. Three days later, cells were plated on 10-cm dishes and cultured until reaching a confluent state. After G418 selection, clones were subjected to Western blot analysis with a specific anti-V5 antibody (Invitrogen, Carlsbad, CA, USA).
For cell proliferation assay, 6 000 cells were seeded per well in triplicate in 96-well plates in normal growth media. After 24-h (d1) and 72-h (d3) incubation, cell proliferation assay was performed using cell proliferation ELISA, BrdU (5-bromo-2-deoxyuridine) kit (Roche Diagnostics) according to the manufacturer’s instructions. Cell proliferation ratio at d3 was normalized by that of d1. For cell viability assay, 10 000 cells were seeded per well in triplicate in 96-well plates in normal growth media. After 24-h incubation, cells were incubated with gemcitabine at various concentrations. After 48-h incubation, cell viability was measured by using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Cells were treated with MTT solution at a concentration of 0.5 mg/mL for 2 h, and then solubilized in dimethylsulfoxide (DMSO). Color reaction was measured by a spectrometer at a wavelength of 570 nm. Each experiment was repeated at least twice.
Apoptosis was determined by staining with Hoechst. Cells were plated at 1×104 on culture slide (Becton Dickinson, Franklin Lakes, NJ, USA) and allowed to adhere to the slide overnight. Gemcitabine (1 μg/mL) was added and incubated for an additional 24 h. The cells were fixed with 40 g/L paraformaldehyde for 20 min, followed by Hoechst 33342 (Calbiochem-Novabiochem, La Jolla, CA, USA) staining in these cells for 30 min. Then the cells were analyzed under a fluorescence microscope (Leica, Cambridge, UK). Between the incubations, the specimens were washed thrice with PBS. Chromatin condensation, nuclear shrinkage, and nucleosomal fragmentation were considered to be morphological markers of apoptosis.
APOCYTO Caspase-3 Colorimetric Assay Kit (MBL, Nagoya, Japan) was used for the detection of caspase-3 activity following the manufacturer’s protocol. The subconfluent state cells in 10-cm dishes were harvested after 24-h incubation with gemcitabine (1 µg/mL), and subjected to caspase-3 activity detection.
Cells were lysed by the addition of lysis buffer containing 150 mmol/L NaCl, 50 mmol/L Tris–HCl, 10 mL/L Nonidet P40 and 5 g/L sodium deoxycholate. Cell lysates were cleared by centrifugation at 16 000 g at 4 ˚C for 15 min. Cleared lysates were boiled for 5 min at 100 ˚C after the addition of 5× sample loading buffer containing 1 mol/L Tris–HCl (pH 6.8), sodium dodecyl sulfate, glycerol, and bromophenol blue. Samples were electrophoresed at 200 V on 125 g/L polyacrylamide gels and transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA, USA), blocked with 50 g/L nonfat dry milk, and then incubated with primary antibodies, such as anti-caspase-3 antibody (BD610322), anti-v5 antibody (R960-25, Invitrogen) and anti-α-tubulin antibody (sc-8035, Santa Cruz Biotechnology, Santa Cruz, CA, USA). Horseradish peroxidase-conjugated anti-mouse antibody (NA931, Amersham, Buckinghamshire, UK) was used as secondary antibody. Reactive bands were detected using ECL™ Western Blotting Detection Reagents (Amersham).
The unpaired t-test and one-way ANOVA were used for statistical comparison. Calculations were made with the help of Microsoft Excel computer software (Microsoft, Redmond, WA, USA). P<0.05 was considered statistically significant.
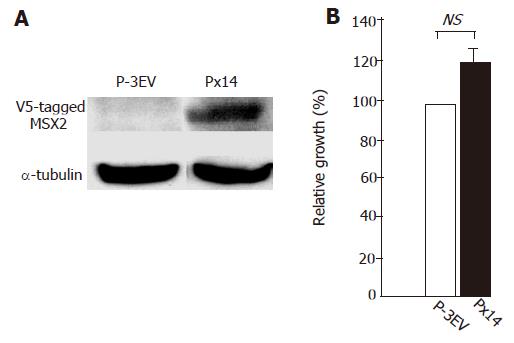
To evaluate whether MSX2 expression correlates with gemcitabine resistance in pancreatic cancer cells, we generated MSX2 stable transfectant cell line. As described previously (Hamada et al, submitted), V5-tagged MSX2 expression vector was transfected in Panc-1 cells. MSX2-expressing clone (Px14) was selected and subjected to further analysis (Figure 1A). Panc-1 cells transfected with empty vector were maintained with the culture media containing G418, and used as control cell line (P-3EV). BrdU incorporation assay showed no significant difference in cell growth between two cell lines, but there was a tendency of slightly increased proliferation ratio in Px14 cells (P = 0.074, Figure 1B) relative to control cells (P-3EV cells), which was compatible to our previous report (Satoh et al, submitted).
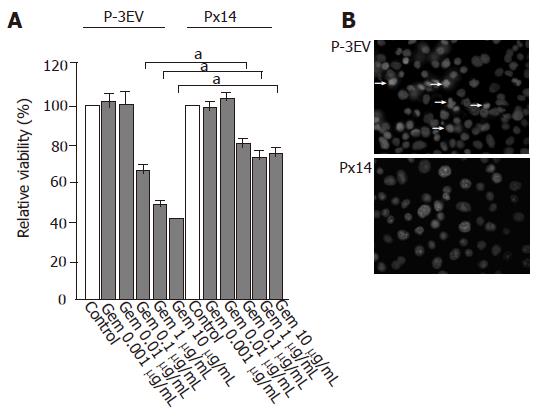
Gemcitabine administration at a concentration of 10 µg/mL for 48 h decreased the viability of P-3EV cells by 60%, whereas that of Px14 cells only by 30% (Figure 2A). Px14 cells also depicted gemcitabine resistance at concentrations of 1 and 0.1 µg/mL (Figure 2A). Since Px14 cells were thought to be resistant to gemcitabine cytotoxicity, we analyzed gemcitabine-induced morphological alteration in P-3EV cells and Px14 cells. Gemcitabine-treated P-3EV cells at a concentration of 1 µg/mL for 24 h showed obvious apoptotic characteristics such as nuclear fragmentation and chromatin condensation, while these morphological changes were unremarkable in Px14 cells (Figure 2B).
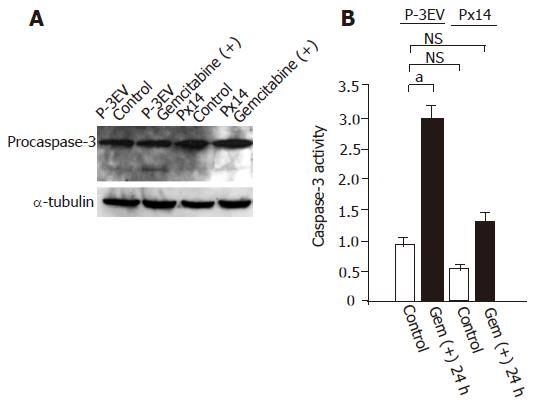
Gemcitabine treatment led P-3EV cells to apoptotic state, thus we examined caspase-3 activation in P-3EV and Px14 cells. Gemcitabine administration at a concentration of 1 µg/mL clearly induced active (cleaved) form of caspase-3 in P-3EV cells, but not in Px14 cells (Figure 3A). Caspase-3 activation is a critical event in apoptosis induction; therefore, we hypothesized that gemcitabine resistance in Px14 cells might be due to suppressed caspase-3 activity. To evaluate this hypothesis, we conducted direct measurements of caspase-3 activities in P-3EV cells and Px14 cells, with or without gemcitabine treatment. The basal activities of caspase-3 in these cells were not significantly different (Figure 3B). However, gemcitabine treatment at a concentration of 1 µg/mL significantly increased caspase-3 activity in P-3EV cells but not in Px14 cells as compared with its basal activity in control cells.
Even in pancreatic cancer cells, apoptotic signal does exist, but the signal is considered to be overwhelmed by inhibitor of apoptosis[10]. In our experiment, gemcitabine treatment induced apoptotic changes in P-3EV cells, whereas this effect was attenuated by MSX2 overexpression. Studies have shown that the ability of evasion from apoptosis is one of the critical steps for tumor progression. For example, interference of X-linked inhibitor of apoptosis (XIAP) expression in MDA-MB-231 mammary cancer cell line increases sensitivity to several chemotherapeutic agents[11]. The expression level of survivin in pancreatic cancer tissue is significantly associated with the reduction of the apoptotic index of tumor cells[12] and is also reported to be correlated with the prognosis of patients[13], indicating that anti-apoptotic effectors directly affect clinical outcomes.
MSX2 overexpression enhanced cell proliferation in pancreatic cancer cell line BxPC-3 (Satoh K et al, submitted), and tended to stimulate the Panc-1 cell growth in the current study, indicating that MSX2 enhances the proliferation effect rather than the pro-apoptotic effect in pancreatic cancer cells. Association of MSX2 with cell proliferation has also been shown in facial mesenchyme[14] and in skeletogenic mesenchyme[15], suggesting that the downstream targets of this homeobox gene may include regulators of cell proliferation. In addition, MSX2 itself and/or BMP-induced MSX2 has shown to lead the pancreatic cancer cells to epithelial-mesenchymal transition (EMT) state (Satoh et al, Hamada et al, submitted), indicating the enhancement of malignant phenotype of pancreatic cancer cells by MSX2. In this study, we clearly demonstrated that forced expression of MSX2 in pancreatic cancer cells produced the resistance to gemcitabine-induced apoptosis via the suppression of caspase-3 activity, which is an additional new aspect of MSX2 to accelerate the pancreatic cancer cell malignancy. Although further detailed investigations would be required to clarify how MSX2 inhibits caspase-3 activation in pancreatic cancer cells, the involvement of this gene in the development of gemcitabine resistance provides us some clues for further understanding of the cancerous cell nature and a novel therapeutic target.
Science Editor Kumar M and Guo SY Language Editor Elsevier HK
| 1. | Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363:1049-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1481] [Cited by in RCA: 1541] [Article Influence: 73.4] [Reference Citation Analysis (0)] |
| 2. | Chandler NM, Canete JJ, Callery MP. Caspase-3 drives apoptosis in pancreatic cancer cells after treatment with gemcitabine. J Gastrointest Surg. 2004;8:1072-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Schniewind B, Christgen M, Kurdow R, Haye S, Kremer B, Kalthoff H, Ungefroren H. Resistance of pancreatic cancer to gemcitabine treatment is dependent on mitochondria-mediated apoptosis. Int J Cancer. 2004;109:182-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 92] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Hussein SM, Duff EK, Sirard C. Smad4 and beta-catenin co-activators functionally interact with lymphoid-enhancing factor to regulate graded expression of Msx2. J Biol Chem. 2003;278:48805-48814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 175] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 5. | Schwartz DR, Wu R, Kardia SL, Levin AM, Huang CC, Shedden KA, Kuick R, Misek DE, Hanash SM, Taylor JM. Novel candidate targets of beta-catenin/T-cell factor signaling identified by gene expression profiling of ovarian endometrioid adenocarcinomas. Cancer Res. 2003;63:2913-2922. [PubMed] |
| 6. | Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2796] [Cited by in RCA: 2849] [Article Influence: 109.6] [Reference Citation Analysis (0)] |
| 7. | Shtutman M, Zhurinsky J, Simcha I, Albanese C, D'Amico M, Pestell R, Ben-Ze'ev A. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci USA. 1999;96:5522-5527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1701] [Cited by in RCA: 1779] [Article Influence: 68.4] [Reference Citation Analysis (0)] |
| 8. | He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3533] [Cited by in RCA: 3598] [Article Influence: 133.3] [Reference Citation Analysis (0)] |
| 9. | Zhang T, Otevrel T, Gao Z, Gao Z, Ehrlich SM, Fields JZ, Boman BM. Evidence that APC regulates survivin expression: a possible mechanism contributing to the stem cell origin of colon cancer. Cancer Res. 2001;61:8664-8667. [PubMed] |
| 10. | Yang L, Cao Z, Yan H, Wood WC. Coexistence of high levels of apoptotic signaling and inhibitor of apoptosis proteins in human tumor cells: implication for cancer specific therapy. Cancer Res. 2003;63:6815-6824. [PubMed] |
| 11. | McManus DC, Lefebvre CA, Cherton-Horvat G, St-Jean M, Kandimalla ER, Agrawal S, Morris SJ, Durkin JP, Lacasse EC. Loss of XIAP protein expression by RNAi and antisense approaches sensitizes cancer cells to functionally diverse chemotherapeutics. Oncogene. 2004;23:8105-8117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 130] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 12. | Satoh K, Kaneko K, Hirota M, Masamune A, Satoh A, Shimosegawa T. Expression of survivin is correlated with cancer cell apoptosis and is involved in the development of human pancreatic duct cell tumors. Cancer. 2001;92:271-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 13. | Kami K, Doi R, Koizumi M, Toyoda E, Mori T, Ito D, Fujimoto K, Wada M, Miyatake S, Imamura M. Survivin expression is a prognostic marker in pancreatic cancer patients. Surgery. 2004;136:443-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Ashique AM, Fu K, Richman JM. Endogenous bone morphogenetic proteins regulate outgrowth and epithelial survival during avian lip fusion. Development. 2002;129:4647-4660. [PubMed] |
| 15. | Ishii M, Merrill AE, Chan YS, Gitelman I, Rice DP, Sucov HM, Maxson RE. Msx2 and Twist cooperatively control the development of the neural crest-derived skeletogenic mesenchyme of the murine skull vault. Development. 2003;130:6131-6142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 155] [Article Influence: 7.4] [Reference Citation Analysis (0)] |