Published online Oct 28, 2005. doi: 10.3748/wjg.v11.i40.6360
Revised: March 13, 2005
Accepted: March 16, 2005
Published online: October 28, 2005
AIM: To investigate the roles of mucin histochemistry, cytokeratin 7/20 (CK7/20) immunoreactivity, clinical characteristics and endoscopy to distinguish short-segment Barrett’s esophageal (SSBE) from cardiac intestinal metaplasia (CIM).
METHODS: High iron diamine/Alcian blue (HID/AB) mucin-histochemical staining and immunohistochemical staining were used to classify intestinal metaplasia (IM) and to determine CK7/20 immunoreactivity pattern in SSBE and CIM, respectively, and these results were compared with endoscopical diagnosis and the positive rate of gastroesophageal reflux disease (GERD) symptoms and H pylori infection. Long-segment Barrett’s esophageal and IM of gastric antrum were designed as control.
RESULTS: The prevalence of type III IM was significantly higher in SSBE than in CIM (63.33% vs 23.08%, P<0.005). The CK7/20 immunoreactivity in SSBE showed mainly Barrett’s pattern (76.66%), and the GERD symptoms in most cases which showed Barrett’s pattern were positive, whereas H pylori infection was negative. However, the CK7/20 immunoreactivity in CIM was gastric pattern preponderantly (61.54%), but there were 23.08% cases that showed Barrett’s pattern. H pylori infection in all cases which showed gastric pattern was significantly higher than those which showed Barrett’s pattern (63.83% vs 19.30%, P<0.005), whereas the GERD symptoms in gastric pattern were significantly lower than that in Barrett’s pattern (21.28% vs 85.96%, P<0.005).
CONCLUSION: Distinction of SSBE from CIM should not be based on a single method; however, the combination of clinical characteristics, histology, mucin histochemistry, CK7/20 immunoreactivity, and endoscopic biopsy should be applied. Type III IM, presence of GERD symptoms, and Barrett’s CK7/20 immunoreactivity pattern may support the diagnosis of SSBE, whereas non-type III IM, positive H pylori infection, and gastric CK7/20 immunoreactivity pattern may imply CIM.
- Citation: Liu GS, Gong J, Cheng P, Zhang J, Chang Y, Qiang L. Distinction between short-segment Barrett’s esophageal and cardiac intestinal metaplasia. World J Gastroenterol 2005; 11(40): 6360-6365
- URL: https://www.wjgnet.com/1007-9327/full/v11/i40/6360.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i40.6360
The incidence of adenocarcinoma at the distal esophagus and gastric cardia has been increasing rapidly for more than two decades in the Western world, but the adenocarcinoma of distal stomach is decreasing gradually. This phenomenon has so far not been satisfactorily explained. These gastroesophageal junction (GEJ) carcinomas appear to arise from intestinal metaplasia (IM) foci that develop either in the distal esophagus or in the proximal stomach (gastric cardia). Some studies indicate that short-segment Barrett’s esophageal (SSBE) and cardiac intestinal metaplasia (CIM) are precancerous conditions of the adenocarcinoma at GEJ[1,2]. However, the potential carcinogenesis, and the roles in the development of adenocarcinoma at GEJ are probably the differences between them[3]. Some studies have shown that the risk of malignancy is substantially higher for IM in the SSBE than CIM[4], and medical societies, therefore, have recommended endoscopic cancer surveillance routinely for patients with SSBE, but not for patients with CIM[5]. A recent study has shown that biopsy specimens taken from the GEJ frequently show specialized IM, even in patients who have no esophageal symptoms and a normal-appearing distal esophagus[6,7]. Therefore, it is necessary to distinguish SSBE from CIM. However, there are not precise criteria in endoscopy and histology, and reliable methods in distinguishing SSBE from CIM have not yet been established[8]. Ormsby et al[9] once observed a specific pattern of immunoreactivity for cytokeratin 7 and 20 (CK7/20), defined as Barrett’s CK7/20 pattern, which seemed to be distinctive for Barrett’s esophageal, but this method has been disputed by others[10,11].
In this study, we used high iron diamine/Alcian blue (HID/AB) mucin-histochemical methods and CK7/20 immunoreactivity, combined with clinical characteristics and endoscopic appearances, to diagnose SSBE and CIM strictly, and long-segment Barrett’s esophageal (LSBE) and intestinal metaplasia of gastric antrum (GA-IM) were designed as control. We aimed to investigate the roles of these methods in distinguishing SSBE from CIM.
The study group consisted of 118 patients who underwent endoscopies because of a variety of upper gastrointestinal complains. All patients were required to record complete clinical, histological, and endoscopic data.
Clinical data included age, gender, complains, and symptoms of gastroesophageal reflux disease (GERD), which included heartburn, reflux acid, and regurgitation. The diagnostic criteria of GERD were as follows: there were at least twice of aforementioned symptoms within 1 week, and persisted at least 6 months, and all symptoms could be relieved significantly with H2 receptor blocker or proton pump inhibitor.
Histological data required presence of IM, which was proved with hematoxylin–eosin (HE) staining, and excluded dysplasia or adenocarcinoma.
Endoscopic data included precise biopsy location, precise site of squamocolumnar junction (SCJ) and GEJ, and presence or absence of hiatus hernia.
The patients who were classified into four groups according to histological and endoscopic data are as follows:
LSBE with IM: Twenty-eight patients with IM in LSBE, which was defined as 3 cm or more of columnar mucosa in the esophagus. Biopsies were taken from reddish island-like or long tongue-like columnar mucosa in distal esophagus.
SSBE with IM: Thirty patients with IM in SSBE, which was defined endoscopically as tongues less than 3 cm in length above GEJ with its resemblance to small intestine having well-formed microvilli, and biopsies were taken from red, velvet-like columnar mucosa in distal esophagus.
CIM: Twenty-six patients with SCJ coincided precisely with GEJ, and biopsies were obtained within 2 cm below GEJ.
GA-IM: Thirty-four patients who underwent biopsies from gastric antrum.
On the other hand, eight normal distal esophagus mucosa and cardiac mucosa were selected as normal controls, which were defined as such by the combination of a normal endoscopic appearance of the mucosa and the absence of acute or chronic inflammation, IM, and dysplasia or adenocarcinoma.
Each endoscopic biopsy was sliced into three sections with a thickness of 4-5 μm to carryout histological examination. Biopsies with IM were stained with HID/AB to identify neutral mucins, sialomucins, and sulfomucins. Briefly, slides were immersed into HID solution for more than 18-24 h (includes 6–8 h in warm-box at 60 °C). Slides were then stained with 10 g/L Alcian blue solution (pH 2.5) for 30 min. Finally, the slides were stained with 10 g/L neutral red solution for 2 min.
Slides were deparaffinized and rehydrated through graded alcohols. Antigen retrieval was performed by using citrate buffer in a microwave, both for CK7 and CK20 at 92-96 °C. Slides were incubated in 30 mL/L hydrogen peroxide/methanol for 20 min to block nonspecific background staining due to endogenous peroxidase. Using the standard streptavidin–biotin–peroxidase complex (ABC) method, the slides were incubated with primary antibodies CK7 (1:50 dilution) and CK20 (1:30 dilution). The slides were further incubated for 30 min in a secondary antibody solution. Diaminobenzidine served as the chromogen. The slides were counterstained with hematoxylin. The samples of breast carcinoma and colon carcinoma were used as positive controls for CK7 and CK20, respectively. Negative controls were produced with the same tumor samples and staining methods by omitting the primary antibodies.
Giemsa staining was used to assess H pylori infection.
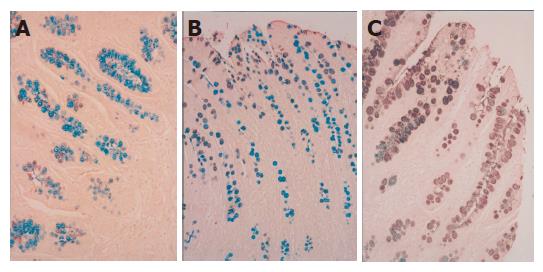
Classification of IM IM was classified into three subtypes as previously described by El-Zimaity et al[12]: complete type (type I); incomplete small intestinal type (type II); and incomplete colonic type (type III). Type I: non-secretory absorptive cells and sialomucin-secreting goblet cells. Type II: few absorptive cells, columnar cells secreting neutral and acid sialomucin, and goblet cells secreting mainly sialomucin but occasionally sulfomucin. Type III: columnar cells secreting predominantly sulfomucin and goblet cells secreting sialomucin or sulfomucin. The HID/AB method stains sialomucins blue and sulfomucin brown (Figure 1). Normal colonic mucosa served as a positive control for this stain. Type I was classified as complete IM, and type II and III were grouped into incomplete IM.
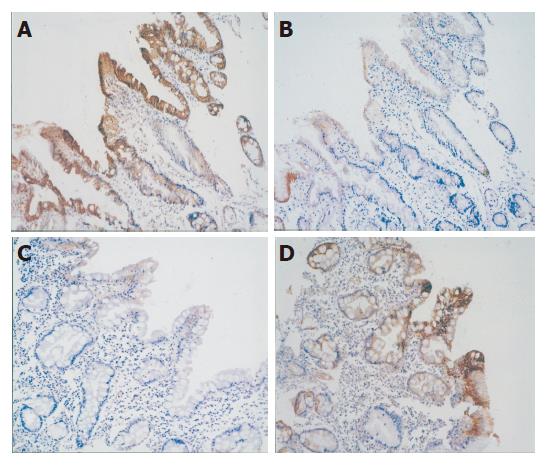
Three types of CK7/20-staining patterns were recognized in areas of IM, similar to those described by Ormsby et al[9]. Barrett’s CK7/20 pattern was defined as staining of both superficial and deep metaplastic epithelium for CK7, and staining of the superficial epithelium for CK20 (Figures 2A and B). Gastric CK7/20 pattern that depended on the histochemical type of IM are as follows: complete IM was characterized by absence of CK7 immunostaining and strong diffuse CK20 immunostaining (Figures 2C and D), whereas incomplete IM was characterized by weak patchy CK7 staining and moderate patchy CK20 immunostaining. All the patterns that did not belong to Barrett’s or gastric pattern were defined as other patterns.
Using software SPSS 10.0 for Windows, statistical analyses were performed. The comparison of prevalence at different sites was performed using χ2 test. A P value <0.05 was considered statistically significant.
The classifications of IM at different sites are shown in Table 1.
The prevalence of type III IM in SSBE was 63.33%, similar to LSBE (75.00%), but significantly higher than CIM (23.08%) and GA-IM (17.65%, P<0.005). However, there was no statistical difference between CIM and GA-IM. On the contrary, the preponderant subtype of IM in CIM and GA-IM were type I and type II. These results indicated that the LSBE and SSBE mainly contained type III subtypes of IM, whereas there were low incidence of type III in CIM and GA-IM.
The CK7/20 immunoreactivity patterns at different sites are shown in Table 2.
There were significant differences in CK7/20 immunoreactivity staining among LSBE, SSBE, CIM, and GA-IM (P<0.005). The CK7/20 immunoreactivity pattern in SSBE was mainly Barrett’s pattern (73.33%), similar to LSBE (78.57%). On the contrary, the preponderant CK7/20 immunoreactivity pattern in CIM was gastric pattern (57.69%), similar to GA-IM (70.59%), but there were still 26.92% cases in CIM, which showed Barrett’s pattern. These results implied that although there was a significant difference in CK7/20 immunoreactivity pattern between SSBE and CIM, there were also several cases, which did not belong to either Barrett’s pattern or gastric pattern. Therefore, CK7/20 immunoreactivity pattern alone could not distinguish all SSBE and CIM reliably.No difference in CK7/20 immunoreactivity pattern was observed between types II and III IM at any site. However, all cases with type I IM were stained with gastric CK7/20 immunoreactivity pattern.
Comparing the differences between the expression of CK7/20 immunoreactivity pattern and the positive prevalence of GERD and H pylori infection at different sites of IM, comprehensively, we observed that the positive prevalence of GERD in the cases with Barrett’s CK7/20 pattern was significantly higher than those with gastric CK7/20 pattern. On the contrary, the positive prevalence of H pylori infection in the cases with gastric CK7/20 pattern was significantly higher than those with Barrett’s CK7/20 pattern. Furthermore, the symptoms of GERD in the cases with LSBE and SSBE that showed Barrett’s pattern were all positive, whereas the H pylori infection in 60% (6/10) cases that showed gastric pattern was positive. H pylori infection in all the cases that showed gastric pattern in CIM was positive, and accompanied with chronic atrophic gastritis, whereas the symptoms of GERD in six cases with Barrett’s pattern were all positive. These results implied that LSBE and SSBE were probably associated with GERD, whereas CIM was mainly associated with H pylori infection, but the CK7/20 immunoreactivity pattern showing Barrett’s pattern in CIM was probably associated with GERD. Furthermore, these results suggested that the CK7/20 immunoreactivity pattern could be used to distinguish CIM caused by GERD from that was caused by H pylori infection.
IM may develop in GEJ in patients either with SSBE or carditis, which is defined as CIM. Distinction between these two entities is important, since the etiology and risk of developing adenocarcinoma are different[13,14]. SSBE is believed to be caused by GERD, and associated with an increased risk of esophageal adenocarcinoma, similar to LSBE[15-17], whereas the etiology and clinical importance of CIM are unclear. Therefore, it is necessary to distinguish them from each other. However, because there are not precise criteria used to diagnose SSBE and CIM in histology, as well as in endoscopy, it is not so easy to distinguish them precisely.
The best criterion for the distinction between SSBE and CIM is the precise knowledge that the biopsy specimens are from the esophagus or the stomach, but because of the effects of the anatomic variation of GEJ and SCJ, inflammation and hiatus hernia, this is not always possible in endoscopy. In histological morphology, there is also no significant difference between SSBE and CIM. Although few scholars have reported that the areas adjacent to CIM show normal foveolar epithelium, whereas those of Barrett's epithelium contain pre-goblet cells that can be stained positive by Alcian blue method[18], these characteristics do not exist in all biopsy specimens. Therefore, it is not reliable to distinguish SSBE from CIM histologically. HID/AB staining have also been used to distinguish SSBE from CIM. IM at GEJ (or ultra-short-segment Barrett’s esophagus) has been more frequently found to express sulfomucins, which is defined as type III in classification of IM, and to involve the surface glandular epithelium than CIM[19,20]. Piazuelo et al[21] also found that the area covered with incomplete type of IM and the proportion of sulfomucins were significantly higher in the esophagus compared to the stomach. In our study, we found that the prevalence of type III IM in SSBE was 63.33%, similar to LSBE, but significantly higher than CIM (23.08%). Therefore, we think that the HID/AB method could be used to distinguish SSBE from CIM initially, based on the different expressions of neutral mucins, sialomucins, and sulfomucins.
Cytokeratins (CK) are a family of at least 20 structural proteins found in the cytoskeletons of epithelial cells. Certain epithelia exhibit characteristic patterns of CK expression depending on the type, location, and differentiation of the epithelium. CK7, essentially, is not expressed in normal epithelium of the gastrointestinal tract, whereas CK20 is expressed in intestinal epithelium, gastric foveolar epithelium, and endocrine cells in the upper portions of the pyloric glands[22]. Ormsby et al[9] observed a pattern of immunoreactivity for CK7 and CK20 that seemed to be distinctive for Barrett’s esophagus, which was defined as staining of the superficial epithelium for CK20 and staining of both superficial and deep metaplastic epithelium for CK7. This so-called “Barrett’s CK7/20 pattern” was found in 100% esophageal biopsy specimens from LSBE, but none of the specimens of IM were from the stomach. Ormsby et al[23] later found that this Barrett’s CK7/20 pattern was present in 82% of patients with SSBE. Studies by Sarbia et al[24], Glickman et al[20] and Jovanovic et al[25] confirmed findings of Ormsby et al[9] in 90.3%, 91%, and 94% of their cases with LSBE, respectively. However, some other studies did not support the findings of Ormsby et al[9]. In addition, Mohammed et al[10] and by El-Zimaity et al[11] demonstrated that the proposed Barrett’s CK7/20 pattern was observed only in 54% and 39% of patients with LSBE, respectively. Differences in patient populations and endoscopic biopsy techniques may have contributed to the discrepancies among these studies, especially, the sites of the biopsies. Previous anatomic and endoscopic studies have shown the gastric cardia to be a small structure varying from about 0 to 10 mm in length (mean length 3 mm) beneath the GEJ[26], but there are no agreement on the precise distal extent of the cardia[5]. In our study, we have defined the distal extent of the cardia as less than 2 cm beneath the GEJ, which is accepted by most scholars at present, and applied an anterograde approach to obtain biopsies by avoiding biopsy across the SCJ, aiming to eliminate biopsy error to most extent. On the other hand, we compared the results of SSBE and CIM with LSBE and GA-IM. The results indicated that 73.33% of SSBE showed Barrett’s CK7/20 immunoreactivity pattern, which was similar to LSBE; whereas 57.69% of CIM showed gastric CK7/20 pattern, which was similar to GA-IM. These results implied that CK7/20 immunoreactivity pattern could be used to distinguish most of SSBE from CIM. However, there were a large number of cases, which belonged to neither Barrett’s pattern nor gastric pattern, since they showed some controversial results; therefore, we have simply based our study on the CK7/20 immunoreactivity pattern, and could not distinguish all SSBE from CIM.
The etiology and pathogenesis of SSBE and CIM are unclear until recently. Recent data have suggested the cause of SSBE, like that of LSBE, is probably related to chronic GERD, and not to H pylori infection[14,27,28]. However, the cause of CIM is still controversial. Schnell et al[29] suggest GERD as a probable cause, whereas Dulai et al[30] have found an association with H pylori infection. Few scholars even think that CIM has dual etiologies: gastroesophageal reflux in some and H pylori infection in others[31,32]. A recent report has described findings in biopsies of the cardia that are said to distinguish between gastroesophageal reflux and H pylori as the etiology[33]. On the basis of these findings, some scholars have proposed that the CK7/20 immunoreactivity patterns with gastroesophageal reflux symptoms and the status of the remainder of the gastric mucosa, including H pylori infection, can distinguish IM caused by GERD from that caused by H pylori infection. Balaji et al[31] found that there were two different patterns of CK7/20 staining in patients with CIM, which support the concept of dual etiologies for CIM. A Barrett's staining pattern was associated with objective evidence of gastroesophageal reflux and the absence of H pylori, suggesting that CK7/20 immunostaining is useful to determine the likely etiology of CIM. Couvelard et al[34] also found that the Barrett’s type CK7/20 pattern was related to a high frequency of gastroesophageal reflux symptoms and normal endoscopic appearance of the stomach, but the gastric type CK7/20 pattern was linked to high frequency of H pylori infection, antral inflammation with atrophy and IM. In the present study, we found that the symptoms of GERD were all positive in some cases of LSBE and SSBE, which showed the Barrett’s type CK7/20 pattern, whereas in other cases that showed gastric CK7/20 pattern, the symptoms of GERD were all negative, but H pylori infection was positive conversely. In contrast, in the CIM cases that showed gastric type CK7/20 pattern, H pylori infection was all positive, and the symptoms of GERD were mostly negative, whereas in other cases which showed Barrett’s type, the symptoms of GERD were all positive. These results indicate partly that the CK7/20 immunoreactivity patterns, combined with gastroesophageal reflux symptoms and statuses of H pylori infection, could imply the probable cause of SSBE and CIM, and even help to distinguish from each other. However, although GERD symptoms were significantly more common among the patients with IM showing a Barrett’s CK7/20 pattern, Couvelard et al[34] also found that most of those patients (57%) had no symptoms of GERD. Conversely, although H pylori infection was found significantly more often in gastric biopsy specimens from patients with IM showing a gastric CK7/20 pattern, 34% of those patients had no demonstrable H pylori infection. Our findings are also in agreement with the above results. Thus, the concept that CK7/20 immunoreactivity patterns can distinguish IM caused by GERD from that caused by H pylori infection is appealing, and further investigations are needed to resolve this dispute.
Therefore, on the basis of our results, we conclude that the distinction of SSBE from CIM should not be based on a single method or factor or characteristic, but the combination of clinical characteristics, histological results, mucin-histochemistry, CK7/20 immunoreactivity, and especially precise endoscopic biopsy. Type III IM, presence of GERD symptoms and Barrett’s CK7/20 immunoreactivity pattern may support the diagnosis of SSBE, whereas non-type III IM, presence of H pylori infection and gastric CK7/20 immunoreactivity pattern may support the diagnosis of CIM.
We thank Professor Wei Ding, Department of Pathology, the First Affiliated Hospital, Medical College of Zhejiang University, for gifting us the reagent of HID/AB.
Co-first-authors: Gui-Sheng Liu and Jun Gong
Co-correspondent: Gui-Sheng Liu
Science Editor Kumar M and Guo SY Language Editor Elsevier HK
| 1. | Ruol A, Parenti A, Zaninotto G, Merigliano S, Costantini M, Cagol M, Alfieri R, Bonavina L, Peracchia A, Ancona E. Intestinal metaplasia is the probable common precursor of adenocarcinoma in barrett esophagus and adenocarcinoma of the gastric cardia. Cancer. 2000;88:2520-2528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 2. | Cameron AJ, Souto EO, Smyrk TC. Small adenocarcinomas of the esophagogastric junction: association with intestinal metaplasia and dysplasia. Am J Gastroenterol. 2002;97:1375-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Goldblum JR. The significance and etiology of intestinal metaplasia of the esophagogastric junction. Ann Diagn Pathol. 2002;6:67-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Sharma P, Weston AP, Morales T, Topalovski M, Mayo MS, Sampliner RE. Relative risk of dysplasia for patients with intestinal metaplasia in the distal oesophagus and in the gastric cardia. Gut. 2000;46:9-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 127] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 5. | Spechler SJ. The role of gastric carditis in metaplasia and neoplasia at the gastroesophageal junction. Gastroenterology. 1999;117:218-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 111] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Law S, Lam KY, Chu KM, Wong J. Specialized intestinal metaplasia and carditis at the gastroesophageal junction in Chinese patients undergoing endoscopy. Am J Gastroenterol. 2002;97:1924-1929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Hirota WK, Loughney TM, Lazas DJ, Maydonovitch CL, Rholl V, Wong RK. Specialized intestinal metaplasia, dysplasia, and cancer of the esophagus and esophagogastric junction: prevalence and clinical data. Gastroenterology. 1999;116:277-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 338] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 8. | Morales CP, Spechler SJ. Intestinal metaplasia at the gastroesophageal junction: Barrett's, bacteria, and biomarkers. Am J Gastroenterol. 2003;98:759-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Ormsby AH, Goldblum JR, Rice TW, Richter JE, Falk GW, Vaezi MF, Gramlich TL. Cytokeratin subsets can reliably distinguish Barrett's esophagus from intestinal metaplasia of the stomach. Hum Pathol. 1999;30:288-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 146] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 10. | Mohammed IA, Streutker CJ, Riddell RH. Utilization of cytokeratins 7 and 20 does not differentiate between Barrett's esophagus and gastric cardiac intestinal metaplasia. Mod Pathol. 2002;15:611-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | El-Zimaity HM, Graham DY. Cytokeratin subsets for distinguishing Barrett's esophagus from intestinal metaplasia in the cardia using endoscopic biopsy specimens. Am J Gastroenterol. 2001;96:1378-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 55] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | El-Zimaity HM, Ramchatesingh J, Saeed MA, Graham DY. Gastric intestinal metaplasia: subtypes and natural history. J Clin Pathol. 2001;54:679-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 93] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Wang LD, Zheng S, Zheng ZY, Casson AG. Primary adenocarcinomas of lower esophagus, esophagogastric junction and gastric cardia: in special reference to China. World J Gastroenterol. 2003;9:1156-1164. [PubMed] |
| 14. | Goldblum JR, Vicari JJ, Falk GW, Rice TW, Peek RM, Easley K, Richter JE. Inflammation and intestinal metaplasia of the gastric cardia: the role of gastroesophageal reflux and H. pylori infection. Gastroenterology. 1998;114:633-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 176] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 15. | Haggitt RC. Barrett's esophagus, dysplasia, and adenocarcinoma. Hum Pathol. 1994;25:982-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 441] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 16. | Blot WJ, Devesa SS, Fraumeni JF. Continuing climb in rates of esophageal adenocarcinoma: an update. JAMA. 1993;270:1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 198] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 17. | Haggitt RC. Adenocarcinoma in Barrett's esophagus: a new epidemic? Hum Pathol. 1992;23:475-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 75] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Mueller J, Werner M, Stolte M. Barrett's esophagus: histopathologic definitions and diagnostic criteria. World J Surg. 2004;28:148-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Hackelsberger A, Günther T, Schultze V, Manes G, Dominguez-Muñoz JE, Roessner A, Malfertheiner P. Intestinal metaplasia at the gastro-oesophageal junction: Helicobacter pylori gastritis or gastro-oesophageal reflux disease? Gut. 1998;43:17-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 106] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Glickman JN, Wang H, Das KM, Goyal RK, Spechler SJ, Antonioli D, Odze RD. Phenotype of Barrett's esophagus and intestinal metaplasia of the distal esophagus and gastroesophageal junction: an immunohistochemical study of cytokeratins 7 and 20, Das-1 and 45 MI. Am J Surg Pathol. 2001;25:87-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 92] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Piazuelo MB, Haque S, Delgado A, Du JX, Rodriguez F, Correa P. Phenotypic differences between esophageal and gastric intestinal metaplasia. Mod Pathol. 2004;17:62-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Kurtkaya-Yapicier O, Gencosmanoglu R, Avsar E, Bakirci N, Tozun N, Sav A. The utility of cytokeratins 7 and 20 (CK7/20) immunohistochemistry in the distinction of short-segment Barrett esophagus from gastric intestinal metaplasia: Is it reliable? BMC Clin Pathol. 2003;3:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Ormsby AH, Vaezi MF, Richter JE, Goldblum JR, Rice TW, Falk GW, Gramlich TL. Cytokeratin immunoreactivity patterns in the diagnosis of short-segment Barrett's esophagus. Gastroenterology. 2000;119:683-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Sarbia M, Donner A, Franke C, Gabbert HE. Distinction between intestinal metaplasia in the cardia and in Barrett's esophagus: the role of histology and immunohistochemistry. Hum Pathol. 2004;35:371-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Jovanovic I, Tzardi M, Mouzas IA, Micev M, Pesko P, Milosavljevic T, Zois M, Sganzos M, Delides G, Kanavaros P. Changing pattern of cytokeratin 7 and 20 expression from normal epithelium to intestinal metaplasia of the gastric mucosa and gastroesophageal junction. Histol Histopathol. 2002;17:445-454. [PubMed] |
| 26. | Takubo K, Mafune K, Tanaka Y, Sasajima K. Pathology of the cardia. Nihon Geka Gakkai Zasshi. 1998;99:547-551. [PubMed] |
| 27. | Goldblum JR. Inflammation and intestinal metaplasia of the gastric cardia: Helicobacter pylori, gastroesophageal reflux disease, or both. Dig Dis. 2000;18:14-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Csendes A, Smok G, Quiroz J, Burdiles P, Rojas J, Castro C, Henríquez A. Clinical, endoscopic, and functional studies in 408 patients with Barrett's esophagus, compared to 174 cases of intestinal metaplasia of the cardia. Am J Gastroenterol. 2002;97:554-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Schnell TG, Sontag SJ, Chejfec G, Aranha G, Metz A, O'Connell S, Seidel UJ, Sonnenberg A. Long-term nonsurgical management of Barrett's esophagus with high-grade dysplasia. Gastroenterology. 2001;120:1607-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 388] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 30. | Dulai GS, Guha S, Kahn KL, Gornbein J, Weinstein WM. Preoperative prevalence of Barrett's esophagus in esophageal adenocarcinoma: a systematic review. Gastroenterology. 2002;122:26-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 243] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 31. | Balaji NS, DeMeester SR, Wickramasinghe KS, Hagen JA, Peters JH, DeMeester TR. Etiology of intestinal metaplasia at the gastroesophageal junction. Surg Endosc. 2003;17:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | DeMeester SR, Wickramasinghe KS, Lord RV, Friedman A, Balaji NS, Chandrasoma PT, Hagen JA, Peters JH, DeMeester TR. Cytokeratin and DAS-1 immunostaining reveal similarities among cardiac mucosa, CIM, and Barrett's esophagus. Am J Gastroenterol. 2002;97:2514-2523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 33. | Griffel LH, Amenta PS, Das KM. Use of a novel monoclonal antibody in diagnosis of Barrett's esophagus. Dig Dis Sci. 2000;45:40-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Couvelard A, Cauvin JM, Goldfain D, Rotenberg A, Robaszkiewicz M, Fléjou JF. Cytokeratin immunoreactivity of intestinal metaplasia at normal oesophagogastric junction indicates its aetiology. Gut. 2001;49:761-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 65] [Article Influence: 2.7] [Reference Citation Analysis (0)] |