Published online Oct 28, 2005. doi: 10.3748/wjg.v11.i40.6312
Revised: December 21, 2004
Accepted: December 23, 2004
Published online: October 28, 2005
AIM: To investigate their expression and activity in the rat ileum after exposure to ionizing radiation along with that of the cellular effectors and molecular mediators involved in the regulation of MMPs.
METHODS: Rats were exposed to a single 10-Gy dose of X-rays delivered to the abdomen. A combination of methods, such as zymography, immunohistochemistry and real time reverse transcriptase-polymerase chain reaction, were used to localize and quantify MMPs and the molecules involved in MMP activating and inhibitory pathways (plasmin/plasminogen, TIMPs), CD8+, as well as inflammatory (interleukin (IL)-1β, IL-8, tumor necrosis factor-α, TNF-α) and fibrogenic mediators (transforming growth factor-β1-3) within ileal tissue at 1, 3, and 7 d after irradiation.
RESULTS: A marked increase in MMP-2 and -14 mRNA and protein levels associated with an increased activity of MMP-2 was observed in irradiated ileal tissue. MMP-2 and -14 expression was mainly observed in inflammatory, epithelial, and mesenchymal cells, whereas a slight increase in MMP-3 expression was detected in the few infiltrating macrophages at d 1 after irradiation. Conversely, MMP-1, -7, and -9 mRNA levels were not found to be affected by abdominal irradiation. Irradiation was found to induce disappearance of CD8+ cells. Furthermore, we have observed that TNF-α and IL-1β protein levels increased 6 h after irradiation, whereas those of IL-8 only increased after 3 d and was concomitant with neutrophil infiltration. In addition, the expressions of molecules involved in MMP activating and inhibitory pathways (urokinase-type plasminogen activator and tissue-type plasminogen activator; TIMP-1, TIMP-2, and plasminogen activator-inhibitor-1) were found to be increased after abdominal irradiation.
CONCLUSION: This study showed that abdominal irradiation induces an acute remodeling of the ileum associated with an increased expression of MMPs and TIMPs that do not involve CD8+ T cells but involve mesenchymal and epithelial cells, although to a lesser extent, and probably even soluble inflammatory and fibrogenic mediators.
- Citation: Strup-Perrot C, Vozenin-Brotons MC, Vandamme M, Linard C, Mathé D. Expression of matrix metalloproteinases and tissue inhibitor metalloproteinases increases in X-irradiated rat ileum despite the disappearance of CD8a T cells. World J Gastroenterol 2005; 11(40): 6312-6321
- URL: https://www.wjgnet.com/1007-9327/full/v11/i40/6312.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i40.6312
Acute intestinal toxicity may occur in response to therapeutic or accidental exposure to ionizing radiation. At the histopathological level, it is characterized by a decrease in the depth of the intestinal crypts and the height of the villi. It is associated with basement membrane degradation, and ultimately leads to mucosal barrier breakdown and ulceration. Classical radiation-induced toxicity symptoms include diarrhea, malabsorption, and protein losing enteropathy[1]. Depending on the delivered dose of radiation, a restoration phase involving desquamation and re-epithelialization may occur. This requires extracellular matrix (ECM) remodeling, a process in which proteases and especially matrix metall-oproteinases (MMPs) play an essential role[2], and which is associated with the overexpression of inflammatory and fibrogenic cytokines, such as interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α) and transforming growth factor-β (TGF-β)[3,4].
Cell adhesion, migration, proliferation, and differentiation are required for complete wound healing and rely on interactions between cells and the ECM. Normal wound ECM interactions are therefore essential to wound healing. The rate, quality/effectiveness, and organization of this process are determined by a dynamic balance between overall matrix synthesis, deposition, and degradation. Disruption of this balance likely induces abnormal matrix degradation or accumulation. MMPs are a large family of zinc-dependent matrix degrading enzymes, involved in the fine tuning of ECM homeostasis. MMPs are classified, based on their substrate specificity and structural features, into six categories: gelatinases (MMP-2 and -9), stromelysins (MMP-3, -10, and -11), elastases (MMP-12), collagenases (MMP-1, -8, -13, and -18), matrilysins (MMP-7 and -26) and membrane-type MMPs (MMP-14, -15, -16, and -17)[2]. The primary regulatory mechanism of MMP activity occurs at the transcriptional level and consists of a variety of extracellular signals involving cytokines, growth factors, and cell杕atrix interactions[5,6]. MMPs are then secreted as zymogens, which require prior proteolytic activation. In vivo activation of pro-MMPs is mainly mediated through the plasminogen杙lasmin system, but MMPs themselves may also be involved. The third level of regulation is ensured by the physiological inhibitors of MMPs, which include tissue inhibitors of metalloproteinases (TIMPs). Four subtypes of TIMPs (TIMP 1-4) have been identified so far[7]. TIMP-1 inhibits several MMPs, while TIMP-2 seems to specifically inhibit MMP-2.
Overexpression of MMPs has been known to occur in both physiological and pathological conditions and to involve tissue restoration and/or destruction. Many studies reported that the alteration of ECM remodeling and MMP/TIMP expression occur in inflammatory bowel diseases[8-12]. Furthermore, some studies showed that T cells play a central role in the activation of MMPs in inflammatory bowel diseases[13,14]. However, few studies have examined the role of MMPs in radiation-induced gastrointestinal disorders. After intraoperative irradiation, Seifert et al[15], observed a prolonged gelatinolytic activity in rat colonic anastomoses. Two clinical studies conducted in patients reported controversial data. Hovdenak et al[16], observed increased MMP-2 and MMP-9 expression after irradiation, while Kumar et al[17], did not. In this study, we aimed at confirming that observations made on gelatinases also applied to other types of MMPs. We investigated the expression of MMPs after abdominal X-irradiation and their activating and inhibitory systems (i.e., plasminogen/plasmin and TIMP-1 and -2). We simultaneously investigated the stimulatory signals and focused on the expression of pro-inflammatory (TNF-α, IL-1β) and fibrogenic cytokines (TGF-β) in the ileum after irradiation. Finally, we investigated the involvement of CD8a+T cells in MMP activation after irradiation. The results presented in this study show that irradiation induces a moderate inflammatory state, a significantly increased expression of MMP-2, MMP-14, TIMP-1, and TIMP-2, and the disappearance of CD8a+T cells.
Experiments were performed using male Wistar rats (Janvier, Le Genest Saint Isle, France), weighing initially between 225 and 250 g. Prior to irradiation, rats were anesthetized by groups of six with 2.5% isoflurane, administered at a rate of 0.4 L/min (Abbott, Rungis, France). A protective lead screen (5 mm thick) was placed over each animal to cover them from the top of the head to 1 cm below the ribs. The given dose was 10 Gy at a rate of 0.62 Gy/min (Phillips, 250 MeV, 0.2-mm Cu filter). Sham-irradiated rats were placed within the apparatus and anesthetized, but were not exposed to X-rays. Animals were weighed and food intake was measured 10 d before exposure to radiation and daily during the week following irradiation. All experiments were conducted according to the French regulations on animal experimentation (Ministry of Agriculture, Act No. 87-848, October 19, 1987).
One, three, and seven days after irradiation, rats were anesthetized with 2.5% isoflurane and the terminal ileum was removed before euthanasia (sodium pentobarbitone, 100 mg/kg, ip; Sanofi, La Ballastière, France) and rinsed with sterile physiological saline. The ileum was cut into three equal pieces immediately after resection: The tissue samples were formalin-fixed for histology, frozen in liquid nitrogen, and crushed into powder for RNA and protein extraction.
Samples were fixed in 40 g/L formaldehyde (Carlo Erba, Rueil Malmaison, France) for 3 d at room temperature. They were then dehydrated, paraffin-embedded, and cut into 4-μm-thick sections. Slides were stained with hematoxylin, eosin, and saffron for histological analysis.
MMP and TIMP antibodies were purchased from Chemicon (Euromedex, Mundolshiem, France) and used as follows: anti-MMP-1 (41-1E5, 1:15 000), anti-MMP-2 (42-5D11, 1:200), anti-MMP-3 (SL-1 IIIC4, 1:200), anti-MMP-7 (ID-2, 1:300), anti-MMP-14 (113-5B7, 1:500), anti-TIMP-1 (102B1, 1:50), and anti-TIMP-2 (67-4H11, 1:1 000). The anti-myeloperoxidase antibody was from Novocastra (Téu, Le Perray en Yvelines, France; NCL MYELOp, 1:300) and the anti-CD8a antibody was from Cedarlane (Téu; CL004AP, 1:600).
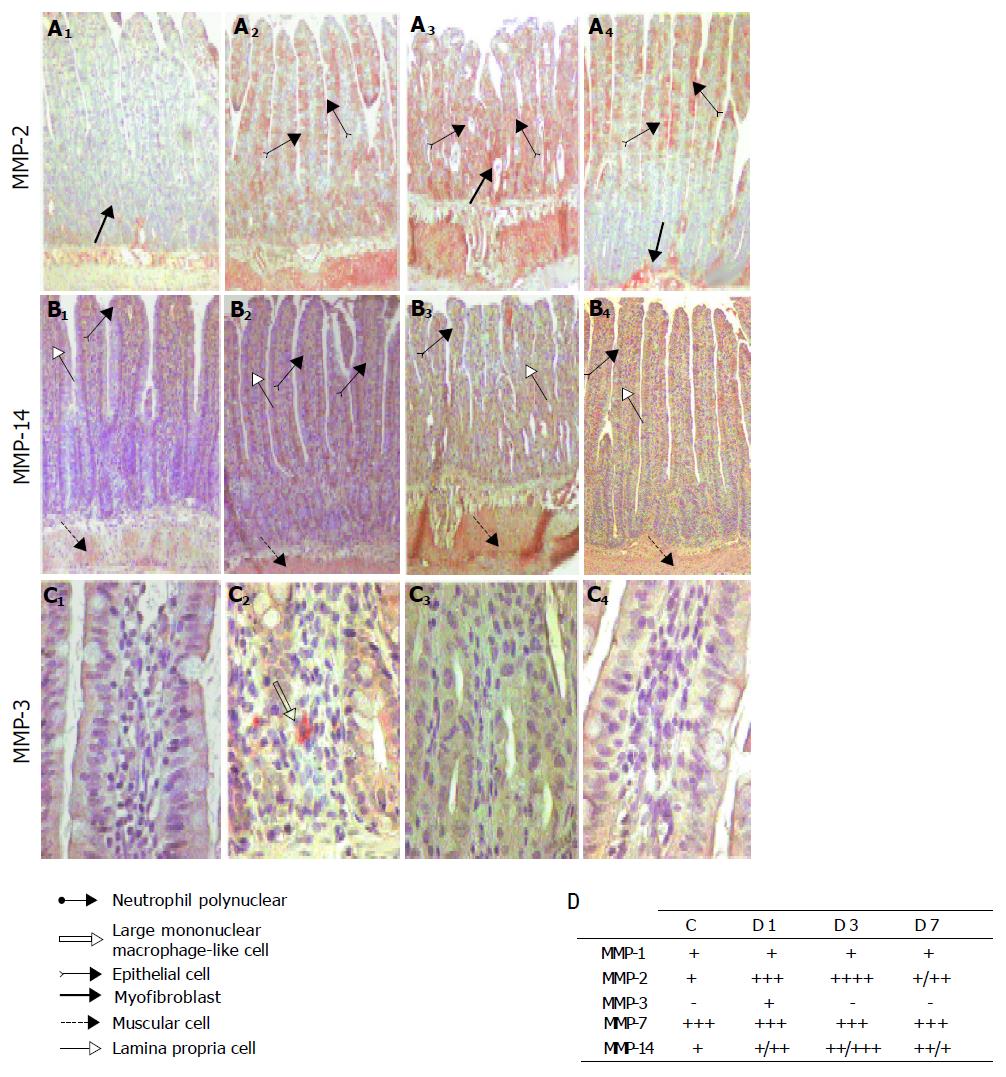
Following deparaffinization and rehydration, endogenous peroxidase activity was inhibited with 30 mL/L hydrogen peroxide in PBS. In order to detect MMP-3, MMP-7, and CD8a epitopes, sections were placed in 0.01 mol/L hot citrate buffer (pH 6.0). The protein block serum-free blocking solution (Dako, Trappes, France) was used to inhibit non-specific staining. Primary antibodies were diluted in antibody diluent (Dako). Antibodies against MMP-1, MMP-2, and TIMP-1 were detected using the LSAB2-HRP system (Dako), whereas antibodies against CD8a, MMP-3, MMP-7, MMP-14, and TIMP-2 were detected using the StrepABC-HRP system (Dako) for myeloperoxidase. Immunostaining was performed using the Vector NovaRED substrate kit for peroxidase (Biovalley, Conches, France, for Vector Laboratories, USA). Sections were counterstained with differentiated Mayer’s Hemalum (Merck for VWR, Fontenay-sous-bois, France). Slides were rinsed between each stage with Tris HCl-NaCl-Tween (50 mmol/L, 0.3 mol/L, 1 g/L). Control staining, without the primary antibody and using an irrelevant mouse IgG, was performed concomitantly with each immunostaining to ensure staining specificity. A semiquantitative analysis of MMP-2, MMP-3, MMP-14, TIMP-1, and TIMP-2 expression was performed. Mean staining intensity scores, which reflected staining intensity in the epithelium, lamina propria, submucosa, blood vessels, and muscularis propria, were attributed. The following scoring system was used: no staining (-); weak staining intensity (+); moderate staining intensity (++); strong staining intensity (+++); and very strong staining intensity (++++).
Crushed tissue samples were homogenized in 50 mmol/L Tris-HCl buffer (pH 7.6) containing 150 mmol/L NaCl, 10 mmol/L CaCl2, 10 g/L Triton X-100, and protease inhibitors (Sigma-reverse Aldrich, St. Quentin Fallavier, France). Protein concentration was determined using the Lowry method.
Zymography was performed as previously described[18]. Briefly, samples (4 μg protein) and MMP-2/9 standards (CC073 Chemicon, Euromedex, Mundolsheim, France) were separated by electrophoresis on 80 g/L SDS-polyacrylamide gels copolymerized with 1 g/L gelatin (Type A from porcine skin; Sigma-Aldrich, St. Quentin Fallavier, France). Gels were washed in 25 g/L Triton X-100, incubated in a buffer containing 50 mmol/L Tris–HCl (pH 7.8), 5 mmol/L CaCl22H2O, 50 mmol/L NaCl, 0.1 g/L Brij 35, and 0.2 g/L NaN3 at 37 °C, stained with 5 g/L Coomassie blue in 250 mL/L isopropanol/100 mL/L acetic acid, and destained in a 100 mL/L methanol/100 mL/L acetic acid solution. Gelatinolytic bands appeared as clear zones against the blue background. Gelatinases were identified by their molecular weight and after inhibition using 20 mmol/L EDTA or 1 mmol/L o-phenanthroline. Densitometric analyses were performed using an imaging workstation (Biocom, Les Ulis, France) interfaced with the Phoretix image analysis software (Nonlinear Dynamics, Newcastle upon Tyne, UK).
Total RNA was extracted from crushed tissue samples by homogenization in 4 mol/L guanidine isothiocyanate, purified using the method of Chomczynski and Sacchi and quantified by spectrophotometry (A260/A280). RNA was treated with RNase-free DNase (0.5 U/μL) to remove contaminating genomic DNA. RNA integrity was assessed by denaturing agarose-gel electrophoresis and staining with ethidium bromide.
Real-time reverse transcriptase-polymerase chain reaction (RT-PCR) was used to quantify the levels of MMP-2, -9, -14, TIMP-1, -2, plasminogen, urokinase-type plasminogen activator (u-PA), tissue-type plasminogen activator (t-PA), plasminogen activator-inhibitor-1 (PAI-1), IL-8, TGF-β1, TGF-β2, and TGF-β3 RNA transcripts as previously described[19]. Primers were generated with the Primer Express software (Applied Biosystems, Courtaboeuf, France) and purchased from Invitrogen (Cergy-Pontoise, France): MMP-2 5’-ACCGTCGCCCATCATCAA-3’ (forward), 5’-CCTTCAGCACAAAGAGGTTGC-3’ (reverse); MMP-9 5’-TGTCCAGACCAAGGGTACAGC-3’ (forward), 5’-GAAGAATGATCTAAGCCCAGCG-3’ (reverse); MMP-14 5’-GAGGGTCATGAGAAGCAGGC-3’ (forward), 5’-TCAAAGGGTGTGCTGTCGC-3’ (reverse); TIMP-1 5’-AGAAGGGCTACCAGAGCGATC-3’ (forward), 5’-ATCGAGACCCCAAGGTATTGC-3’ (reverse); TIMP-2 5’-CTACATCTCCTCCCCGGATGA-3’ (forward), 5’-GGTGCCCATTGATGCTCTTC-3’ (reverse); plasminogen 5’-CTGAGTATCTAAACAACAGAGTCAAATCC-3’ (forward), 5-TCGAAGCAAACCAGAGGTCC-3’ (reverse); PAI-1 5’-ATGGCTCAGAACAACAAGTTCAAC-3’ (forward), 5’-CAGTTCCAGGATGTCGTACTCG-3’ (reverse); t-PA 5’-GACGTGAAGCCCTGGTGC-3’ (forward), 5’-CAAGCCGGCGTGCTG-3’ (reverse); u-PA 5’-GTTTGAGGTGGAGCAGCTCAT-3’ (forward), 5’-GCTATGTCATTATGGAAGGCCAG-3’ (reverse); TNF-α 5’-ATCCGAGATGTGGAACTGGC-3’ (forward), 5’-CGATCACCCCGAAGTTCAGTA-3’ (reverse); TGF-β1 5’-AGTCCCAAACGTCGAGGTGA-3’ (forward), 5’-CCATGAGGAGCAGGAAGGG-3’ (reverse); TGF-β2 5’-TGCTGAGAACCTTTTTGCTCC-3’ (forward), 5’-GTCGAGGGTGCTGCAGGTA-3’ (reverse); TGF-β3 5’-CAAGCAGCGCTACATAGGTGG-3’ (forward), 5’-CAGTGACATCGAAGGACAGCC-3’ (reverse); IL-8 5’-GACTGTTGTGGCCCGTGAG-3’ (forward), 5’-CCGTCAAGCTCTGGATGTTCT-3’ (reverse), and IL-1β 5’-CAACAAAAATGCCTCGTGC-3’ (forward), 5’-TGCTGATGTACCAGTTGGG-3’ (reverse).
Crushed tissue was weighed and homogenized in 10 mmol/L phosphate buffer (pH 7.4) supplemented with protease inhibitors, such as 2 mmol/L PMSF, 10 μg/mL pepstatin A, 1 μg/mL aprotinin, 10 μg/mL leupeptin, and 0.5 mg/mL EDTA (Sigma-Aldrich, St. Quentin Fallavier, France). Protein concentration was measured using a modified version of the Bradford method (Bio-Rad Laboratories, Marne-la-Coquette, France). Concentration of IL-1β and TNF-α was determined by ELISA (R&D Systems, Minneapolis, MN, USA). All data were expressed in picogram per milligram of protein.
Each real-time RT-PCR and cytokine immunoassay were done in six animals and results were expressed as mean±SE. The ANOVA and Student-Newman-Keuls test were used to determine whether the difference between the values obtained with the control group and the irradiated group was statistically significant. P value <0.05 was considered statistically significant.
Abdominal X-irradiation (10-Gy) induced the inhibition of food intake and decrease in body weight. Diarrhea was observed between d 3 and 5 after irradiation.
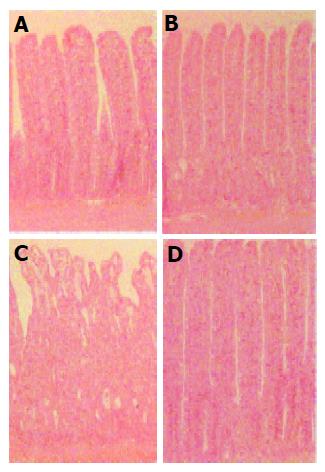
Histopathological changes after irradiation are shown in Figure 1. No significant histological changes were seen 24 h after irradiation, whereas a marked alteration of the intestinal mucosa was observed 3 d after irradiation. A significant decrease in the thickness of the mucosa (-15%, P<0.01), the sloughing of epithelial cells at the top of the villi, associated with an atrophy of the villi, dilation of the crypts, and absence of mucus secretion were observed. Muscle layers, on the other hand, appeared normal. Seven days after irradiation, the ileal epithelium seemed to be regenerated. We observed a significant increase in the thickness of the mucosa of irradiated mice compared to controls (+14%, P<0.01), with an increase in the number of epithelial cells located in the villi. Some crypts were still dilated at this time.
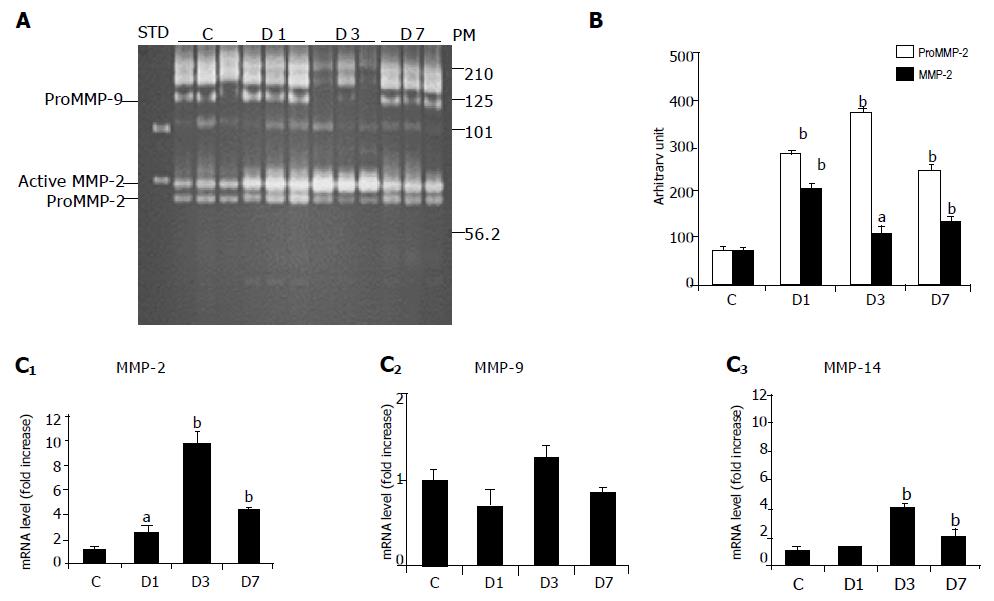
Gelatin zymography allowed the detection of both active and pro-forms of gelatinases (Figure 2A). Equal amounts of pro- (72 ku) and active (62 ku) MMP-2 were detected in control ileal tissues (Figure 2B). The pro-MMP-2 band significantly increased from d 1-7 (×3.8 on d 1; ×5.0 on d 3; ×4.0 on d 7, all P<0.01) after irradiation. Induction of active MMP-2 peaked on d 1 (×2.9, P<0.01) and was still higher than control values on d 3 (×1.5, P<0.05) and 7 (×1.8, P<0.01). We observed a weak gelatinolytic band with a molecular weight, which corresponded to that of pro-MMP-9, but as it was not inhibited by EDTA, it could not be attributed to MMP-9. The high molecular weight bands (125 and 200-220 ku) were, on the other hand, completely inhibited by EDTA and phenanthroline. These gelatinolytic activities, which might have MMP-9 dimers or an MMP-9-lipocalin complex, were equivalent on d 1 and 7 after irradiation, but weaker on d 3 (Figure 2B).
Irradiation-induced transcriptional activation of MMP-2 was confirmed by real-time RT-PCR. MMP-9 mRNA levels, however, were not significantly altered (Figure 2C). Since MMP-14 is involved in the activation of MMP-2, we studied the alterations in MMP-14 mRNA levels. We found that MMP-14 was induced 3 and 7 d after irradiation (Figure 2C).
Immunohistochemistry was used to investigate the cell types that expressed gelatinase after irradiation. A weak MMP-2 staining restricted to the pericryptal sheath myofibroblasts, inflammatory cells, and smooth muscle cells was observed in the control ileal tissues (Figures 3A and B). A significant transmural increase in MMP-2 staining was, however, found on d 1 after irradiation in smooth muscle and epithelial cells, and on d 3 in smooth muscle cells, epithelial cells of the villi, and in pericryptal sheath myofibroblasts. MMP-2 staining decreased on d 7.
In control samples, MMP-14 staining was observed in epithelial cells at the top of the villi, inflammatory cells in the lamina propria, and smooth muscle cells. As for mRNA, in irradiated samples, MMP-14 staining was unchanged on d 1, but had increased on d 3 and had spread to the whole tissue. On d 7, however, MMP-14 staining in irradiated and control samples was equivalent except in epithelial cells at the top of the villi, which exhibited weak MMP-14 staining (Figures 3A and B).
Furthermore, we focused on the expression and immunolocalization of MMP-1, -3, and -7. MMP-1 and -7 were found to be expressed in control ileal tissue, and no changes were observed after irradiation (Figure 3B). MMP-3 was not detected in control samples, but staining was observed at d 1 in some infiltrating mononuclear cells, which were morphologically similar to macrophages (Figures 3A and B).
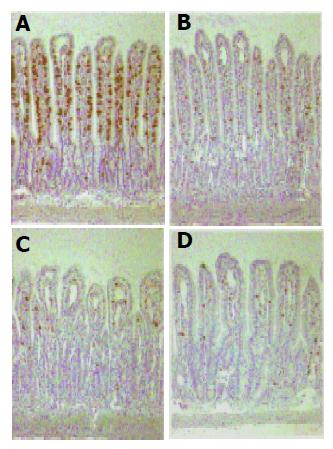
To investigate whether T cells could be involved in MMP activation after X-irradiation, a subset of peripheral T cells, the majority of natural killer (NK) cells, and granular intraepithelial leukocytes of the small intestine were stained with an antibody directed against an antigen present on the surface of thymocytes. This antigen is the rat homolog of the human CD8. Figure 4 shows that X-irradiation of the abdomen with a dose of 10 Gy caused a marked decrease in the number of CD8+ cells as early as 1 d after exposure, which was also observed on d 3 and 7.
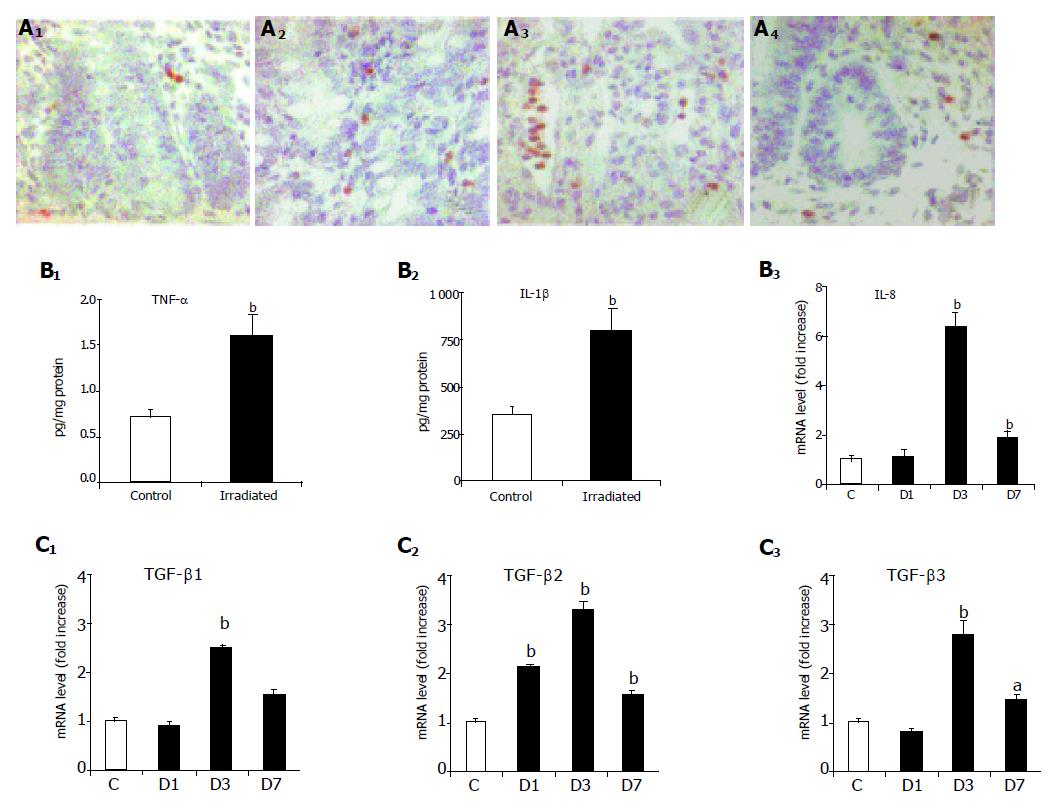
Neutrophil infiltration after abdominal X-irradiation was quantified based on myeloperoxidase staining. While we observed a slight increase in myeloperoxidase staining at d 1 and 3 after irradiation, the number of positive cells returned to control values at d 7 (Figure 5A).
Pro-inflammatory cytokines and fibrogenic growth factors are potent inducers of MMPs expression and activity. Thus, expressions of TNF-α, IL-1β, IL-8, and TGF-β1, -2, and -3 were assessed after X-irradiation. Tissue concentration of pro-inflammatory cytokines TNF-α and IL-1β significantly increased 6 h after irradiation (2.3-fold and 2.3-fold, respectively, P<0.01) as shown in Figure 5B. This early increase was followed by an increase in IL-8 at d 3 (6.4-fold, P<0.01) as shown in Figure 5B.
TGF-β1 and -3 mRNA levels increased 3 d after irradiation (2.5-fold and 1.5-fold, respectively, P<0.01). Furthermore, an increase in TGF-β1 and -3 mRNA levels was also seen on d 7 (2.8-fold, P<0.01; and 1.6-fold, P<0.05, respectively). TGF-β2 mRNA level, on the other hand, increased at d 1 and remained significantly high thereafter (2.1-fold on d 1, 3.2-fold on d 3, and 1.8 fold on d 7, all P<0.01) as shown in Figure 5C.
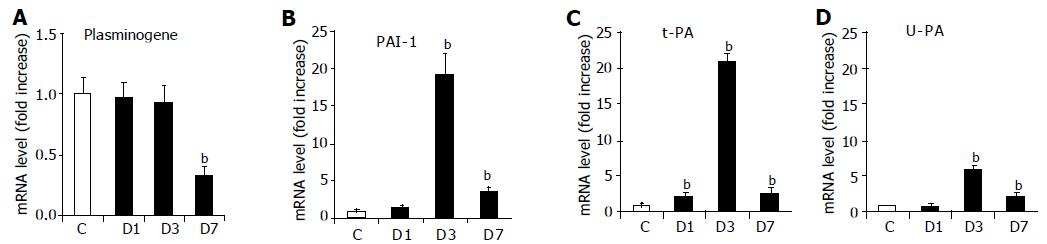
Because the plasminogen system is known to regulate MMP activation, we investigated variations in the mRNA levels of plasminogen, t-PA, u-PA, and PAI-1 after irradiation (Figure 6). Irradiation induced a significant decrease in plasminogen mRNA level on d 7, and a marked increase in u-PA (6.1-fold, P<0.01), t-PA (21-fold, P<0.01), and PAI-1 (19-fold, P<0.01) on d 3, which was also observed on d 7.
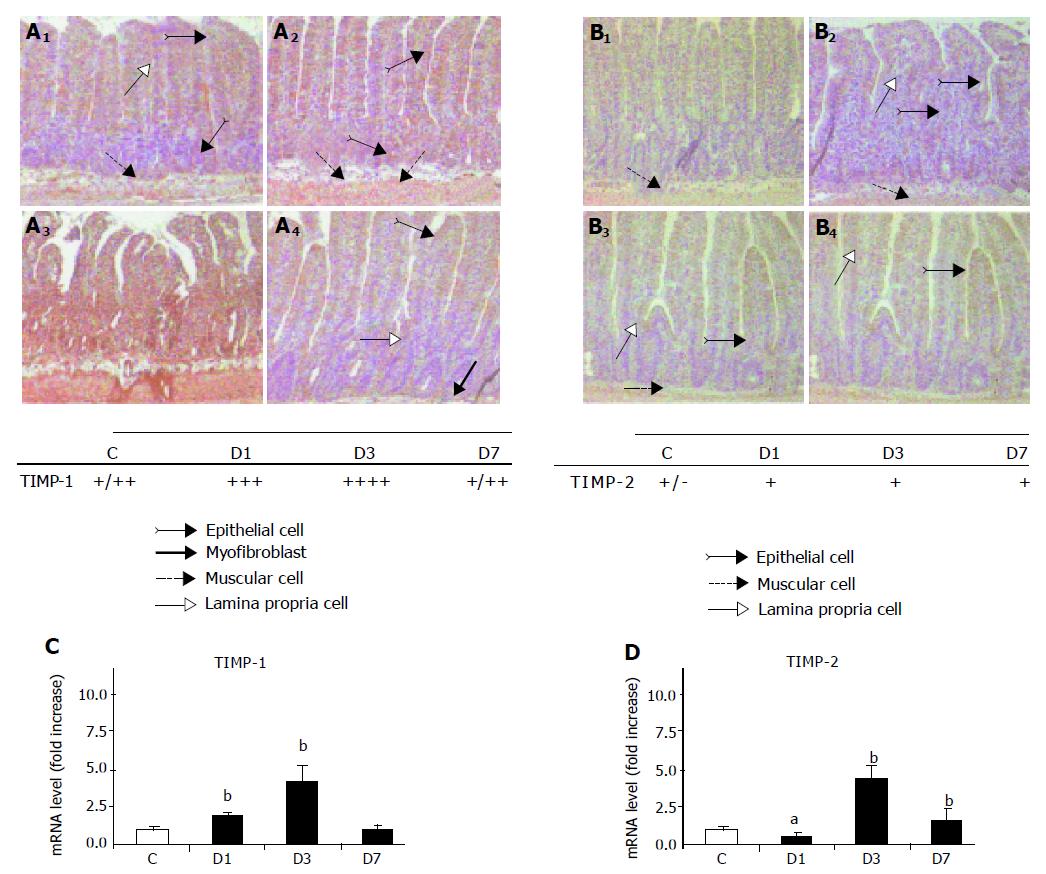
We investigated TIMP-1 and -2 expression, i.e., intensity and localization, in the sections of X-irradiated rat ileum. In control ileal tissue, a moderate TIMP-1 staining was observed in only a few epithelial cells, in inflammatory cells in the lamina propria, and in smooth muscle cells (Figure 7A). TIMP-1 staining increased in all of the layers of the bowel 1 d after irradiation; the most intense staining was observed in epithelial cells. Staining intensity peaked on d 3 after irradiation (Figure 7A). TIMP-1 expression pattern after irradiation was confirmed by the analysis of mRNA level (Figure 7A).
A weak TIMP-2 staining was observed in control ileal tissues (Figure 7B). A slight increase in TIMP-2 staining intensity was observed in epithelial, inflammatory, and smooth muscle cells 1 d after irradiation and could still be observed on d 3 and 7 (Figure 7B). This increase in TIMP-2 mRNA level only reached significance 3 d after irradiation (fourfold increase, P<0.01, Figure 7B).
In this study, we have shown that abdominal X-irradiation with a dose of 10 Gy simultaneously enhances synthesis and activity of MMP-2 and -14. This enhancement is concomitant with an induction of pro-inflammatory cytokines, fibrogenic growth factors, and plasminogen system, which suggests that a complex interplay of factors actually controls radiation-induced activation of MMPs in the intestine. Inhibitory pathways are simultaneously activated as the expression of TIMP-1 and -2 increases, which is suggestive of an irradiation-induced alteration of ECM remodeling. Finally, we observed a decrease in the number of T cells after abdominal irradiation. Our data on radiation-induced induction of gelatinase are in agreement with a previous report by Seifert et al[15], who observed a prolonged gelatinolytic activity in rat colonic anastomoses after intraoperative irradiation. Our results concerning the absence of T cells during radiation-induced ECM remodeling, however, markedly differ from those obtained in models of inflammatory bowel diseases. Our study shows that T lymphocytes are not directly involved in radiation-induced mucosal injuries, suggesting that radiation-induced mucosal breakdown may be promoted by specific cellular pathways and specific molecular signals, which have been investigated further in this study.
Direct evidence of an important T cell-mediated process in the pathogenesis of mucosal lesions has been shown in in vivo and ex vivo models, in which T cells are activated by pokeweed mitogen or anti-CD3 and IL-12 in explants of human fetal intestine[13]. In these models, a severe tissue injury occurs following a massive increase in MMP synthesis (mainly MMP-3 and -9). De Winter et al[22], provided evidence of a cross-talk between lymphocytes and epithelial cells in vivo. This cross-talk is mediated through cytokines that are locally produced by epithelial cells, which regulate immune responses in the intestine. Using the MRC OX8 antibody, specific for T cytotoxic/suppressor cells, granular intraepithelial leukocytes, and NK cells[23], we sought to determine whether T cells were present in the ileal wall. We observed that CD8+ staining completely disappeared 24 h after irradiation, possibly due to radiation-induced apoptosis. Specific target cells are activated after abdominal irradiation, and neutrophils, epithelial, and mesenchymal cells are responsible for the increased expression of MMPs.
Molecular mediators potentially involved in this radiation-induced enhancement of MMP expression were therefore investigated. We focused on the pro-inflammatory cytokines IL-1β and TNF-α as they are seemingly the mediators that are most frequently reported to be involved in bowel inflammatory diseases[24]. In agreement with a previous study[4], levels of IL-β1 and TNF-α increased as soon as 6 h after irradiation, and were returned to control values on d 1, 3, and 7 after irradiation (data not shown). The early increase in IL-β1 and TNF-α may be due to radiation-induced mast-cell degranulation[25]. This increase likely promotes IL-8 induction, leading to leukocyte recruitment and granulocyte infiltration. The radiation-induced inflammatory status, however, appeared to be moderate (twofold increase in TNF-α and IL-β1 protein levels)[4] in comparison to what was reported in Crohn’s disease by Louis et al[11], (10- and 8-fold increase, respectively). This weak inflammatory status may account for the weak MMP-3 stimulation observed in the irradiated ileum. This hypothesis is supported by Mengshol et al[26], who reported that high levels of pro-inflammatory cytokines are required to stimulate MMP-3 secretion by subepithelial myofibroblasts in vitro. Fibrogenic growth factors, such as TGF-β, are also involved in the regulation of cell proliferation, inflammation, immune response, and ECM deposition, which are important parts of the healing process[27,28]. Furthermore, previous studies have suggested that all three TGF-β isoforms are overexpressed during the early phase of radiation-induced injury, while TGF-β1 appears to be predominant in the late phase[29,30]. In this study, we observed an early peak of TGF-β2 induction at d 1, while the other two isoforms (TGF-β1 and TGF-β3) were overexpressed after 3 d, at the onset of the restoration phase. We also observed that induction of MMP-2 mRNA synthesis was concomitant with that of the two TGF-β isoforms. The modulation of MMP-2 expression by TGF-β has already been reported in other models[31,32], and the present results likewise support the hypothesis that the TGF-β family is involved in the regulation of MMP-2 expression after radiation-induced injury.
We investigated the plasminogen-plasmin system, an another activating pathway that is known to regulate MMP activity[2]. This system is a key regulator of fibrinolysis and ECM degradation[33]. Generation of plasmin from plasminogen is regulated by complex interactions between various activators and inhibitors. Classically, plasminogen is known to be activated by u-PA and t-PA. In this study, both u-PA and t-PA were found to be overexpressed 1 d after irradiation, suggesting that the plasmin system is acutely activated after abdominal irradiation. These results are in accordance with the previous reports[34-36] that showed an increase in the fibrinolytic activity in the intestinal mucosa after irradiation. At d 3 and 7, however, the increased expressions of u-PA and t-PA were associated to an increased expression of the inhibitor PAI-1, which may limit plasminogen activation.
Furthermore, we studied the expression of TIMP-1 and TIMP-2, the physiological inhibitors of MMPs. The involvement of TIMPs in inflammatory bowel diseases is still controversial. Heuschkel et al[10], have shown that MMP-3 is overexpressed while TIMP-1 is not. von Lampe et al[37], observed an increase in the level of TIMP-1 mRNA and no modification of the level of TIMP-2 mRNA, suggesting a net increase in the proteolytic activity in inflamed mucosa. Our observations seem consistent with these data obtained in human beings. Induction of TIMP-1 was observed after 1, 3, and 7 d of irradiation, whereas an upregulation of TIMP-2 was seen on d 3 after irradiation. MMP induction rates, however, significantly exceeded TIMP-1 induction. TIMP-2 has been reported to have a bimodal function and to promote activation of MMP-2 when associated with molecules, such as pro-MMP-2 and MMP-14, into large molecular complexes[38]. In this case, synthesis of MMP-14 seems to be the major rate-limiting factor of MMP-2 activation[39]. In this study, expression of both MMP-14 and TIMP-2 was increased after irradiation. Furthermore, MMP-14/TIMP-2 and MMP-2/TIMP-2 mRNA ratios suggested that activation of pro-MMP-2 must have occurred, since a marked increase in the level of active MMP-2 was observed at d 1 after exposure.
In conclusion, the molecular cascades induced by ionizing radiation are complex and are likely to involve more molecules than those studied here. These observations, however, bring new insights on radiation-induced ECM remodeling in the intestine. The fact that CD8a+ cells disappear after abdominal irradiation suggests that specific cellular effectors are involved in radiation-induced activation of MMPs.
Science Editor Kumar M and Guo SY Language Editor Elsevier HK
| 1. | Becciolini A, Balzi M, Potten CS. Radiation effects on proliferation and differentiation in the rat small intestine In. Potten CS, Hendry JH, eds. Radiation and Gut. Amsterdam: Elsevier 1995; 85-143. |
| 2. | Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2922] [Cited by in RCA: 2889] [Article Influence: 120.4] [Reference Citation Analysis (0)] |
| 3. | Burger A, Löffler H, Bamberg M, Rodemann HP. Molecular and cellular basis of radiation fibrosis. Int J Radiat Biol. 1998;73:401-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 89] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Linard C, Ropenga A, Vozenin-Brotons MC, Chapel A, Mathe D. Abdominal irradiation increases inflammatory cytokine expression and activates NF-kappaB in rat ileal muscularis layer. Am J Physiol Gastrointest Liver Physiol. 2003;285:G556-G565. [PubMed] |
| 5. | Nagase H, Woessner JF. Matrix metalloproteinases. J Biol Chem. 1999;274:21491-21494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3230] [Cited by in RCA: 3153] [Article Influence: 121.3] [Reference Citation Analysis (0)] |
| 6. | Pender SL, Salmela MT, Monteleone G, Schnapp D, McKenzie C, Spencer J, Fong S, Saarialho-Kere U, MacDonald TT. Ligation of alpha4ss1 integrin on human intestinal mucosal mesenchymal cells selectively Up-regulates membrane type-1 matrix metalloproteinase and confers a migratory phenotype. Am J Pathol. 2000;157:1955-1962. [RCA] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Baker AH, Edwards DR, Murphy G. Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J Cell Sci. 2002;115:3719-3727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 799] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 8. | Bailey CJ, Hembry RM, Alexander A, Irving MH, Grant ME, Shuttleworth CA. Distribution of the matrix metalloproteinases stromelysin, gelatinases A and B, and collagenase in Crohn's disease and normal intestine. J Clin Pathol. 1994;47:113-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 110] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Baugh MD, Perry MJ, Hollander AP, Davies DR, Cross SS, Lobo AJ, Taylor CJ, Evans GS. Matrix metalloproteinase levels are elevated in inflammatory bowel disease. Gastroenterology. 1999;117:814-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 255] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 10. | Heuschkel RB, MacDonald TT, Monteleone G, Bajaj-Elliott M, Smith JA, Pender SL. Imbalance of stromelysin-1 and TIMP-1 in the mucosal lesions of children with inflammatory bowel disease. Gut. 2000;47:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 110] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Louis E, Ribbens C, Godon A, Franchimont D, De Groote D, Hardy N, Boniver J, Belaiche J, Malaise M. Increased production of matrix metalloproteinase-3 and tissue inhibitor of metalloproteinase-1 by inflamed mucosa in inflammatory bowel disease. Clin Exp Immunol. 2000;120:241-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 110] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Saarialho-Kere UK, Vaalamo M, Puolakkainen P, Airola K, Parks WC, Karjalainen-Lindsberg ML. Enhanced expression of matrilysin, collagenase, and stromelysin-1 in gastrointestinal ulcers. Am J Pathol. 1996;148:519-526. [PubMed] |
| 13. | Salmela MT, MacDonald TT, Black D, Irvine B, Zhuma T, Saarialho-Kere U, Pender SL. Upregulation of matrix metalloproteinases in a model of T cell mediated tissue injury in the gut: analysis by gene array and in situ hybridisation. Gut. 2002;51:540-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 84] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Pender SL, Tickle SP, Docherty AJ, Howie D, Wathen NC, MacDonald TT. A major role for matrix metalloproteinases in T cell injury in the gut. J Immunol. 1997;158:1582-1590. [PubMed] |
| 15. | Seifert WF, Wobbes T, Hoogenhout J, de Man BM, Hendriks T. Intra-operative irradiation prolongs the presence of matrix metalloproteinase activity in large bowel anastomoses of the rat. Radiat Res. 1997;147:354-361. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Hovdenak N, Wang J, Sung CC, Kelly T, Fajardo LF, Hauer-Jensen M. Clinical significance of increased gelatinolytic activity in the rectal mucosa during external beam radiation therapy of prostate cancer. Int J Radiat Oncol Biol Phys. 2002;53:919-927. [RCA] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Kumar A, Collins HM, Scholefield JH, Watson SA. Increased type-IV collagenase (MMP-2 and MMP-9) activity following preoperative radiotherapy in rectal cancer. Br J Cancer. 2000;82:960-965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Kleiner DE, Stetler-Stevenson WG. Quantitative zymography: detection of picogram quantities of gelatinases. Anal Biochem. 1994;218:325-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 646] [Cited by in RCA: 678] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 19. | Strup-Perrot C, Mathé D, Linard C, Violot D, Milliat F, François A, Bourhis J, Vozenin-Brotons MC. Global gene expression profiles reveal an increase in mRNA levels of collagens, MMPs, and TIMPs in late radiation enteritis. Am J Physiol Gastrointest Liver Physiol. 2004;287:G875-G885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | MacDonald TT, Spencer J. Evidence that activated mucosal T cells play a role in the pathogenesis of enteropathy in human small intestine. J Exp Med. 1988;167:1341-1349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 309] [Cited by in RCA: 294] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 21. | Pender SL, Lionetti P, Murch SH, Wathan N, MacDonald TT. Proteolytic degradation of intestinal mucosal extracellular matrix after lamina propria T cell activation. Gut. 1996;39:284-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | De Winter H, Elewaut D, Turovskaya O, Huflejt M, Shimeld C, Hagenbaugh A, Binder S, Takahashi I, Kronenberg M, Cheroutre H. Regulation of mucosal immune responses by recombinant interleukin 10 produced by intestinal epithelial cells in mice. Gastroenterology. 2002;122:1829-1841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Lyscom N, Brueton MJ. Intraepithelial, lamina propria and Peyer's patch lymphocytes of the rat small intestine: isolation and characterization in terms of immunoglobulin markers and receptors for monoclonal antibodies. Immunology. 1982;45:775-783. [PubMed] |
| 24. | Schuppan D, Hahn EG. MMPs in the gut: inflammation hits the matrix. Gut. 2000;47:12-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | MacNaughton WK, Leach KE, Prud'homme-Lalonde L, Ho W, Sharkey KA. Ionizing radiation reduces neurally evoked electrolyte transport in rat ileum through a mast cell-dependent mechanism. Gastroenterology. 1994;106:324-335. [PubMed] |
| 26. | Mengshol JA, Vincenti MP, Coon CI, Barchowsky A, Brinckerhoff CE. Interleukin-1 induction of collagenase 3 (matrix metalloproteinase 13) gene expression in chondrocytes requires p38, c-Jun N-terminal kinase, and nuclear factor kappaB: differential regulation of collagenase 1 and collagenase 3. Arthritis Rheum. 2000;43:801-811. [RCA] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 27. | Dignass AU, Podolsky DK. Cytokine modulation of intestinal epithelial cell restitution: central role of transforming growth factor beta. Gastroenterology. 1993;105:1323-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 316] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 28. | Beck PL, Rosenberg IM, Xavier RJ, Koh T, Wong JF, Podolsky DK. Transforming growth factor-beta mediates intestinal healing and susceptibility to injury in vitro and in vivo through epithelial cells. Am J Pathol. 2003;162:597-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 152] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 29. | Ruifrok AC, Mason KA, Lozano G, Thames HD. Spatial and temporal patterns of expression of epidermal growth factor, transforming growth factor alpha and transforming growth factor beta 1-3 and their receptors in mouse jejunum after radiation treatment. Radiat Res. 1997;147:1-12. [RCA] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Wang J, Zheng H, Sung CC, Richter KK, Hauer-Jensen M. Cellular sources of transforming growth factor-beta isoforms in early and chronic radiation enteropathy. Am J Pathol. 1998;153:1531-1540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 75] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Edwards DR, Murphy G, Reynolds JJ, Whitham SE, Docherty AJ, Angel P, Heath JK. Transforming growth factor beta modulates the expression of collagenase and metalloproteinase inhibitor. EMBO J. 1987;6:1899-1904. [PubMed] |
| 32. | Overall CM, Wrana JL, Sodek J. Transcriptional and post-transcriptional regulation of 72-kDa gelatinase/type IV collagenase by transforming growth factor-beta 1 in human fibroblasts. Comparisons with collagenase and tissue inhibitor of matrix metalloproteinase gene expression. J Biol Chem. 1991;266:14064-14071. [PubMed] |
| 33. | Vassalli JD, Sappino AP, Belin D. The plasminogen activator/plasmin system. J Clin Invest. 1991;88:1067-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 839] [Cited by in RCA: 883] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 34. | Rao JS, Rayford A, Yamamoto M, Ang KK, Tofilon P, Sawaya R. Modulation of fibrinolysis by ionizing radiation. J Neurooncol. 1994;22:161-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Boothman DA, Wang M, Lee SW. Induction of tissue-type plasminogen activator by ionizing radiation in human malignant melanoma cells. Cancer Res. 1991;51:5587-5595. [PubMed] |
| 36. | Sawaya R, Rayford A, Kono S, Ang KK, Feng Y, Stephens LC, Rao JS. Elevated levels of plasminogen activators in the pathogenesis of delayed radiation damage in rat cervical spinal cord in vivo. Radiat Res. 1994;138:386-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 37. | von Lampe B, Barthel B, Coupland SE, Riecken EO, Rosewicz S. Differential expression of matrix metalloproteinases and their tissue inhibitors in colon mucosa of patients with inflammatory bowel disease. Gut. 2000;47:63-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 290] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 38. | Wang Z, Juttermann R, Soloway PD. TIMP-2 is required for efficient activation of proMMP-2 in vivo. J Biol Chem. 2000;275:26411-26415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 285] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 39. | Werb Z. ECM and cell surface proteolysis: regulating cellular ecology. Cell. 1997;91:439-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 989] [Cited by in RCA: 973] [Article Influence: 34.8] [Reference Citation Analysis (0)] |