Published online Oct 28, 2005. doi: 10.3748/wjg.v11.i40.6288
Revised: December 18, 2004
Accepted: December 21, 2004
Published online: October 28, 2005
AIM: To evaluate the multi-step pretargeting radioimm-unoimaging (RII) and radioimmunotherapy (RIT) in nude mice bearing human colon carcinoma with avidin-biotin system labeled with 153Sm.
METHODS: Two- and three-step strategies for avidin-biotin system pretargeting techniques were established. In a three-step procedure, human colon carcinoma bearing nude mice were first injected with biotinylated monoclonal antibody (McAb-Bt) followed by cold avidin (Av) 48 h later and then 153Sm-DB2 24 h thereafter; whereas the two-step procedure consisted of injection of 153Sm-SA 48 h after pretargeting with biotinylated anti-CEA monoclonal antibody (CEA McAb-Bt). SPECT imaging and biodistribution were performed at 4, 24, 48, or 72 h after injection of 153Sm-labeled compounds. Five groups of nude mice subcutaneously grafted with human colon carcinoma were treated 3 d after grafting. One group received the injection with 100 μg CEA McAb-Bt followed by cold avidin (80 μg) after 2 d and 11.1 MBq 153Sm-DB2 after 1 d. Four control groups were treated respectively with 11.1 MBq 153Sm-CEA McAb, 11.1 MBq 153Sm-nmIgG, 11.1 MBq 153Sm-DB2, 100 μL normal saline. Toxicity was evaluated by changes of leukocyte count, and the efficacy by variation in tumor volume. Histological analyses of tumors were performed.
RESULTS: The three-step procedure allowed faster blood clearance and yielded higher tumor blood ratios (5.76 at 4 h and 12.94 at 24 h) of the 153Sm-DB2. The tumor was clearly visualized at 4 h in γ-imaging after the injection of 153Sm-DB2, while a significant accumulation of 153Sm-SA in the tumor was observed only 24 h after the injection and tumor blood ratios at 4 and 24 h were 1.00 and 2.03, respectively, in the two-step procedure. Pretargeting RIT and 153Sm-CEA McAb had a strong tumor-inhibiting effect. The tumor inhibitory rate was 80.67% and 78.44%, respectively, five weeks after therapy. Histopathological evidence also indicated radioactive damage in tumor tissues as necrosis of tumor cells, while in the other organs such as liver and kidney no radioactive damage was observed. Leukocyte counts showed significant decrease after treatment in groups of 153Sm-CEA McAb and 153Sm-nmIgG.
CONCLUSION: The two kinds of pretargeting strategies can elevate the target-to-nontarget ratio, decrease the blood background and shorten the imaging time compared to 153Sm-CEA McAb. Three-step pretargeting RIT is as efficient as 153Sm-CEA McAb, but markedly less toxic. This study provides experimental evidence for the clinical application of pretargeting RII and RIT.
- Citation: Li GP, Zhang H, Zhu CM, Zhang J, Jiang XF. Avidin-biotin system pretargeting radioimmunoimaging and radioimmunotherapy and its application in mouse model of human colon carcinoma. World J Gastroenterol 2005; 11(40): 6288-6294
- URL: https://www.wjgnet.com/1007-9327/full/v11/i40/6288.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i40.6288
Radioimmunoimaging (RII) and radioimmunotherapy (RIT) are nowadays popular research realms in tumor nuclear medicine[1,2]. Various groups have investigated the concept of tumor pretargeting based on the avidin-biotin system[3-6], exploiting the high specificity and strong affinity (Ka = 10-15 mol/L) of avidin (or streptavidin, SA) for biotin, so as to accelerate the elimination of tumor-unbound labeled antibodies from blood and enhance the tumor-to-nontumor (T/NT) ratio. Two-step procedures were thus designed[7]. Paganelli et al[8], have further explored the potential of the avidin-biotin system by devising a three-step procedure, based on the administration of a cold biotinylated antibody, followed by an excess of cold avidin and finally by a radiolabeled biotin derivative. Currently, the pretargeting technique based on avidin-biotin system is widely applied to RII and radioimmunoguided surgery (RIGS) for tumor[9-11]. Avidin-biotin pretargeting RIT is also utilized to treat various tumors and has achieved promising clinical results[12-15].
Samarium-153 (153Sm) is a nuclide that can be produced in high yield and has a high specific activity. It is a β-emitter [Emax = 640 (30%), 710 (50%), and 810 (20%) keV] with a half-life (T1/2) of 1.95 d and 2.5-mm peak path length in tissue and also emits a 103 keV γ-ray that is suitable for γ-camera detection. It possesses excellent property in nuclear physics and chemistry for diagnosis and therapy. Dosimetry estimates can therefore be made by quantification of γ-camera data. Compared to 131I, 153Sm can result in minimal cross irradiation of normal organs and minimal radiation exposure to medical personnel and is a very attractive radioisotope for RII and RIT[16].
We, in this investigation, established the labeling method of SA and biotin with 153Sm, and then the two- and three-step pretargeting RII and three-step RIT were adopted in nude mice bearing human colon carcinoma. The biodistribution and the SPECT imaging were observed and the feasibility of this three-step pretargeting RIT in tumor treatment was also explored.
mAb against carcinoembryonic antigen (anti-CEA McAb) and normal mouse IgG (nmIgG) were prepared by Shanghai Institute of Immunology. BNSH/dimethyl sulfoxide (DMSO) solution was added into bicarbonate buffer solution containing CEA McAb (1 g/L) at a ratio of 15-50:1. The mixture was gently stirred at room temperature for 2 h and finally purified by Sephadex G50 chromatography. Indirect ELISA was performed to assess the activity of biotin, and the purity of biotinylated antibody was determined by SDS-PAGE.
Coupling of cyclic anhydride DTPA (cDTPAa) to anti-CEA McAb or nmIgG was carried out as previously described[19]. Briefly, cDTPAa was suspended in chloroform (1 g/L). An aliquot containing cDTPAa at a molar ratio of 20:1 DTPA:IgG was added to an acid-washed vial and evaporated to dryness under a stream of high-purity dry nitrogen. Anti-CEA McAb or nmIgG (200 μg) was added, the vial was vortexed for 1 min, and then allowed to stand at room temperature for 15-20 min. Acetic acid was added to the mixture to stop the reaction. Separation of the DTPA-CEA McAb or nmIgG conjugate from free DTPA was achieved by Sephadex G50 chromatography. Immunoreactivity of the DTPA-CEA McAb conjugate was assessed using indirect ELISA.
153SmCl3 was supplied by Isotopes Center of China Atomic Energy Institute. SA was purchased from Sigma Chemical Co. (St. Louis, MO, USA). 153SmCl3 at a dose of approximately 40 MBq (with specific activity of 22.2 GBq/mL) was mixed with purified CEA McAb, nmIgG, or SA-DTPA conjugate (0.1 mL) and incubated at room temperature for 20 min. Paper chromatography was carried out, using Xinhua No.1 filter paper (30% ammonium nitrate-treated) as the supporter and the mixture of tributyl phosphate, butanone, and acetic ether (at a proportion of 4:10:3) as the developing agent, to determine the labeling efficiency and radiochemical purity. Immunoreactivity of the labeled McAb was tested using indirect ELISA.
Diethylenetriaminepentaacetic acid d,ω-bis(biocytinamide) (DB2; Sigma Chemical Co., St. Louis, MO, USA) was labeled with 153Sm as follows. 153SmCl3 (37 MBq) was added into 10 μL DB2 solution (2 g/L) for reaction at room temperature for 20 min. Thin-layer chromatography was employed to determine the labeling efficiency and radiochemical purity, using 85% methanol as the developing agent. 153Sm-DB2 had a specific activity of 37 MBq/mL, with a binding capacity to 80% biotin agarose beads.
Balb/c nu/nu mice (female, 20 g) were xenografted subcutaneously in the thigh with 5×106 LoVo cells. Tumor growing to approximately 1 cm in diameter was cut into tiny pieces, which were suspended in normal saline, aspirated and injected (approximately 0.2 mL) subcutaneously into the forelimb of nude mice (4- to 5-wk old). The wounds healed in 12 h. When the tumor grew to a volume of 0.5-1.0 cm3, the mice were used for the subsequent study of pretargeting RII and biodistribution. Therapeutic study was initiated on the 3rd d following tumor inoculation.
Fifty nude mice bearing human colon carcinoma were randomly divided into five groups (three-step group, two-step group, directly labeling group, 153Sm-DB2 group, and 153Sm-SA group), with 10 in each group. In three-step group, the mice were subjected to injections via the tail vein with 100 μg McAb-Bt, then with 30 μg avidin 48 h later, followed by intra-peritoneal (ip) injection of 3.7 MBq (20 μg) 153Sm-DB2. In two-step group, the mice were injected with 100 μg McAb-Bt followed by ip injection of 3.7 MBq (15 μg) 153Sm-SA 48 h later. In directly labeling group, only 153Sm-DTPA-CEA McAb (3.7 MBq, 20 μg) was administrated via ip injection 72 h after the initiation of the experiment. In 153Sm-DB2 group, the mice received only ip injection of 153Sm-DB2 (3.7 MBq, 20 μg). In 153Sm-SA group, the mice received only ip injection of 153Sm-SA (3.7 MBq, 15 μg). Static plane imaging with Siemens ZLC3700 SPECT was performed on the mice of each group at 4, 24, 48, and 72 h, respectively, after the injections. The mice were killed at each time point following the imaging, their organs were isolated and weighed, and the radioactivity counts were determined. The percentage of injected dose per gram of tissue (% ID/g) and T/NT ratio were calculated.
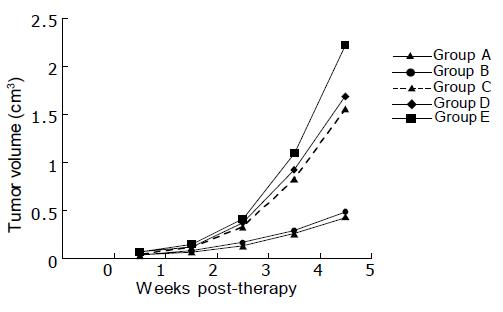
Grouping of the mice Twenty-five tumor-bearing mice were randomly divided into A-E groups (five in each group). The mice in group A received three-step treatment, consisting of injections via the tail vein with 100 μg McAb-Bt, 80 μg Av 48 h later and 11.1 MBq (100 μL) 153Sm-DB2 ip injection after another 24 h. Groups B-D were therapeutic control groups, which were given 153Sm-CEA McAb, 153Sm-nmIgG, and 153Sm-DB2, respectively, at a dose of 11.1 MBq (100 μL). As nontherapeutic control, mice in group E were only given 100 μL of normal saline by ip injection.
Observation of tumor inhibiting effect The length (a) and width (b) of tumor mass were measured double-blindly with a sliding caliper once a week for 5 wk. Tumor volume (V) was calculated according to the formula: V = (1/6) ab2. The inhibition rate (IR) of tumor growth was calculated according to the formula: IR = [(mean tumor volume of nontherapeutic control-mean tumor volume of therapeutic group)/(mean tumor volume of nontherapeutic control)]×100%.
Histological examination Pathological examination was also performed on these mice at the end of the treatment. The mice were killed. Their organs were isolated, weighed, and fixed in a 40 g/L formaldehyde solution, embedded in paraffin, cut into 4-μm-thick sections and then stained with hematoxylin and eosin (HE) for microscopic observation.
Observation of radiation toxicity Toxicity was evaluated by the change of the peripheral WBC counts. The number of WBC of all animals was determined on the day of injection and then once a week for 5 wk consecutively.
Data were expressed as mean±SD and analyzed by Student’s t-test. P<0.05 was considered statistically significant.
In the preparation of McAb-Bt, it was found that one molecule of antibody could conjugate with three molecules of biotin on average at the biotin/McAb molar ratio of 20:1. The resultant McAb-Bt possessed better capability of SA-binding and immunoreactivity. The optimal coupling condition of the McAb and DTPA was as follows: McAb to cDTPAa molar rate of 1:20, pH value of 7-8, and an antibody concentration at 20 mg/mL. The labeling efficiency of 153Sm-CEA McAb was 56-60%, radiochemical purity was over 95% and immunoreactivity was about 50%. The labeling efficiency of 153Sm-DB2 exceeded 80%, and radiochemical purity was over 95% after binding to SA. The labeling efficiency of 153Sm-SA was over 95% and radiochemical purity was over 98%.
The percent of ID/g and T/NT ratio in various organs at different time points during three- and two-step pretargeting are listed in Tables 1 and 2, respectively.
| Group | n | h | Organs | ||||||
| Tumor | Liver | Spleen | Kidney | Bone | Lung | Blood | |||
| Three-step | 3 | 4 | 1.78±0.11 | 1.40±0.16 | 1.60±0.48 | 4.60±2.22 | 0.67±0.27 | 0.27±0.10 | 0.33±0.09 |
| pretargeting | 24 | 1.36±0.17 | 0.92±0.09 | 0.78±0.12 | 5.50±1.55 | 0.58±0.14 | 0.43±0.26 | 0.12±0.06 | |
| 48 | 0.94±0.08 | 0.66±0.12 | 0.61±0.03 | 1.30±0.48 | 0.36±0.02 | 0.17±0.02 | 0.08±0.02 | ||
| Two-step | 3 | 4 | 1.35±0.22 | 3.05±0.07 | 2.31±0.55 | 3.37±0.15 | 0.64±0.12 | 0.50±0.03 | 1.41±0.45 |
| pretargeting | 24 | 2.10±0.16 | 2.02±0.30 | 1.82±0.19 | 4.56±0.42 | 0.33±0.04 | 0.37±0.03 | 1.05±0.13 | |
| 48 | 1.24±0.01 | 1.26±0.01 | 1.26±0.01 | 2.30±0.12 | 0.26±0.01 | 0.27±0.02 | 0.96±0.07 | ||
| Directly | 3 | 4 | 0.54±0.17 | 2.01±0.20 | 1.86±0.21 | 3.21±0.55 | 0.94±0.17 | 0.50±0.10 | 2.08±0.18 |
| labeling McAb | 24 | 0.99±0.16 | 1.53±0.10 | 1.00±0.12 | 2.42±0.24 | 0.61±0.01 | 0.44±0.11 | 1.29±0.48 | |
| 48 | 1.42±0.05 | 1.34±0.04 | 0.77±0.07 | 1.27±0.07 | 0.54±0.07 | 0.34±0.03 | 1.01±0.13 | ||
| 153Sm-DB2 | 3 | 4 | 0.21±0.01 | 1.07±0.10 | 1.14±0.10 | 5.76±0.68 | 0.38±0.04 | 0.37±0.10 | 0.22±0.05 |
| 24 | 0.12±0.02 | 0.83±0.03 | 0.78±0.08 | 3.44±0.24 | 0.24±0.03 | 0.22±0.04 | 0.12±0.01 | ||
| 48 | 0.10±0.01 | 0.62±0.11 | 0.53±0.02 | 2.60±0.84 | 0.14±0.04 | 0.18±0.05 | 0.10±0.02 | ||
| 153Sm-SA | 3 | 4 | 0.32±0.01 | 2.95±0.87 | 2.27±0.46 | 3.79±0.48 | 0.40±0.05 | 0.36±0.12 | 0.94±0.08 |
| 24 | 0.22±0.03 | 1.89±0.01 | 1.82±0.17 | 2.72±0.10 | 0.22±0.01 | 0.33±0.07 | 0.85±0.11 | ||
| 48 | 0.19±0.03 | 1.53±0.16 | 1.38±0.13 | 1.03±0.08 | 0.11±0.01 | 0.20±0.04 | 0.59±0.05 | ||
| Group | n | h | Organs | |||||
| Liver | Spleen | Kidney | Bone | Lung | Blood | |||
| Three-step | 3 | 4 | 1.28±0.18 | 1.21±0.51 | 0.46±0.23 | 2.93±1.05 | 7.12±2.64 | 5.76±1.64 |
| pretargeting | 24 | 1.47±0.11 | 1.76±0.12 | 0.27±0.08 | 2.39±0.27 | 4.06±2.47 | 12.94±5.05 | |
| 48 | 1.44±0.27 | 1.53±0.07 | 0.81±0.40 | 2.64±0.41 | 5.65±1.32 | 11.19±1.80 | ||
| Two-step | 3 | 4 | 0.44±0.08 | 0.60±0.10 | 0.40±0.08 | 2.18±0.60 | 2.71±0.60 | 1.00±0.22 |
| pretargeting | 24 | 1.05±0.15 | 1.16±0.07 | 0.46±0.08 | 6.48±1.30 | 5.78±0.82 | 2.03±0.37 | |
| 48 | 0.98±0.01 | 0.98±0.01 | 0.54±0.02 | 4.77±0.15 | 4.66±0.22 | 1.30±0.08 | ||
| Directly | 3 | 4 | 0.27±0.12 | 0.30±0.12 | 0.17±0.07 | 0.73±0.36 | 1.06±0.16 | 0.26±0.08 |
| labeling McAb | 24 | 0.65±0.11 | 1.01±0.23 | 0.41±0.09 | 1.62±0.27 | 2.31±0.57 | 0.85±0.37 | |
| 48 | 1.07±0.02 | 1.87±0.20 | 1.14±0.13 | 2.71±0.49 | 4.20±0.44 | 1.42±0.13 | ||
| 153Sm-DB2 | 3 | 4 | 0.20±0.01 | 0.19±0.02 | 0.04±0.01 | 0.56±0.04 | 0.61±0.15 | 1.01±0.22 |
| 24 | 0.14±0.02 | 0.15±0.04 | 0.03±0.01 | 0.50±0.14 | 0.55±0.18 | 0.95±0.14 | ||
| 48 | 0.17±0.03 | 0.20±0.01 | 0.04±0.01 | 0.75±0.21 | 0.59±0.17 | 1.04±0.19 | ||
| 153Sm-SA | 3 | 4 | 0.11±0.04 | 0.14±0.03 | 0.08±0.01 | 0.80±0.07 | 0.93±0.22 | 0.34±0.04 |
| 24 | 0.12±0.01 | 0.12±0.02 | 0.08±0.01 | 1.02±0.10 | 0.70±0.24 | 0.26±0.07 | ||
| 48 | 0.13±0.03 | 0.14±0.02 | 0.19±0.04 | 1.77±0.34 | 1.01±0.30 | 0.33±0.02 | ||
As shown in Tables 1 and 2, tumor uptake (% ID/g) was 1.78% and 1.36% in three-step pretargeting group, 4 and 24 h after the injection, and 1.35% and 2.10% in two-step pretargeting group. The tumor/blood ratio at the same time point was 5.76 and 12.94 in three-step pretargeting group, and only 1.00 and 2.03 in two-step pretargeting group. In directly labeling group, the tumor uptake (% ID/g) reached as high as 1.42% 48 h after the injection, but the level of blood background activity was also high (1.01%ID/g). The tumor uptake (% ID/g) and the ratios of tumor to other organs were all lower in 153Sm-DB2 and 153Sm-SA groups.
In the three-step procedure, higher radioactive uptake in the implanted tumor was observed 4 h after the injection, and better tumor display and higher T/NT ratio were also obtained at 24 h because the level of blood background activity was markedly reduced. In the two-step procedure, radioactivity accumulation in the tumor could be visualized at 24 and 48 h. However, higher radioactive uptake was observed in the liver, spleen, and kidney. In the group of directly labeled McAb with 153Sm, radioactive uptake at the site of tumor implantation occurred 72 h after the injection; while in 153Sm-DB2 and 153Sm-SA groups, no significant radioactivity uptake in tumor was observed.
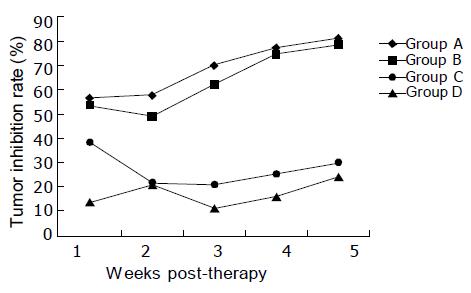
In the 1st wk of treatment, the tumor volume showed little difference among the groups; while in the 2nd wk slower tumor growth rate in groups A and B was observed, indicating tumor inhibition. Till the 5th wk, the tumor volume was obviously smaller in groups A and B than in nontherapeutic control group (Figure 1), and the difference was statistically significant (P<0.01). It was also noted that the tumor volume was smaller in groups C and D than in group E, and the difference was also statistically significant (P<0.01). If the tumor inhibition rate in nontherapeutic control group was considered as zero, the tumor inhibition rate 5 wk post-therapy was as high as 80.67% and 78.44% in groups A and B, much higher than that in groups C and D (Figure 2). In the latter two groups, the tumor inhibition rate was only 29.78% and 23.99%, and no significant difference existed between them. However, significant difference existed between the former two and the latter two groups (P<0.01).
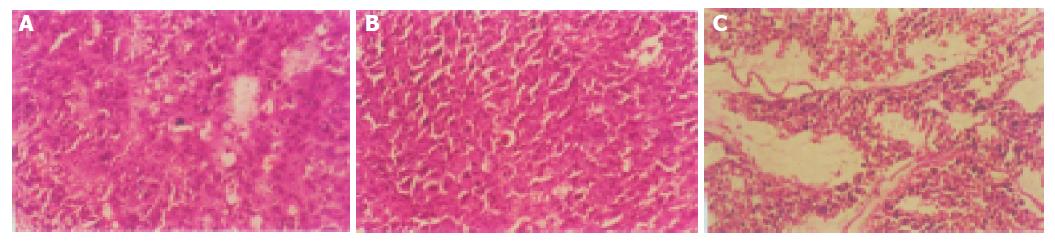
Histopathological evidence also showed that the tumor cell nuclei were pyknotic, karyoclastic, and autolytic in three-step pretargeting RIT and 153Sm-CEA McAb groups, which were not seen in the other controls (Figure 3). In the non-treated group, tumor tissue was characterized by the absence of necrosis, and took on typical forms of carcinoma cells (Figure 3).
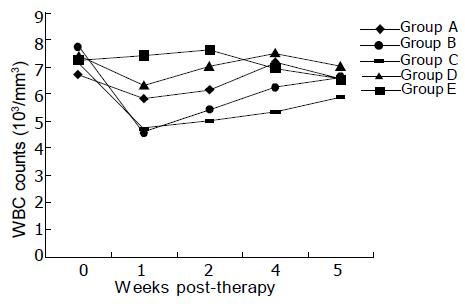
Compared to non-treatment group, the number of peripheral blood WBC in treatment groups decreased after injection of 11.1 MBq of 153Sm to some degree (Figure 4). However, WBC counts showed significant decrease after treatment in 153Sm-CEA McAb and 153Sm-nmIgG groups, and WBC recovery was also slower in both groups. Microscopic observation revealed no obvious tissue radiation damage in organs such as heart, liver, spleen, lungs, and brain in all nude mice at the end of the treatment.
Radiolabeled McAb specific for tumor-associated antigens is used for diagnosis and therapy of malignant tumors[1,2]. However, the blood clearance of antibodies is slow and T/NT ratio of radioactivity in the current system is not high. In order to increase the amount of radioactivity bound to cancer cells, a new approach in cancer therapy called pretargeting has been used. The pretargeting approach can be carried out as a two-step or as a three-step procedure. The two-step procedure is based on the administration of SA-antibody conjugate followed by radioactive biotin or biotinylated antibody is injected followed by radioactive SA[4,18]. In the three-step procedure, biotinylated antibody is injected followed by an excess of avidin/SA and then radioactive biotin is injected[4,19]. In both the methods, the radioactivity of tumor cells is increased[14,20]. Using pretargeting technique of avidin-biotin system, high T/NT ratios have been reported, not only in animal experiments, but also in clinical trials. The avidin-biotin system is also used to reduce the background radioactivity of directly labeled antibody as well as RIGS[9,21]. The success of the approach is due, in part, to the extremely high binding affinity of biotin to avidin/SA, which provides a high tumor targeting efficiency and a long retention at the tumor site. Further, the small size of biotin and its derivatives provides rapid renal clearance of radiolabeled biotin, eliminating much of the irradiation of nontarget tissues. In the present study, the two-step procedure applied consists of injection of radiolabeled SA after pretargeting with biotinylated antibody. The three-step procedure described in our study is as follows. First, tumor pretargeting was done by biotinylated anti-tumor McAbs. Second, when the uptake of McAbs in tumor cells reached its maximum within 24-48 h postinjection, circulating biotinylated McAbs were quickly (5-10 min) removed from the blood by the liver following the administration of avidin as a clearing agent. Finally, postlabeling of the tumor was done by a fast-clearing radioactive biotin and its derivatives. Since one molecule of McAb is capable of binding multiple molecules of biotin, the reaction of avidin with biotin and its derivatives is amplified. One molecule of avidin can bind to four molecules of biotin, and then further amplification of the reaction takes place. In other words, three-step pretargeting technique, like an amplifier, may result in an amplification of signals at the tumor target sites. Meanwhile, transchelating time of radioactive metal in vivo is shortened, and consequently normal tissue uptake of the antibody is reduced and antibody immunoreactivity is preserved unlike directly radiolabeled antibodies, which result in the loss of antibody immunoreactivity due to autoradiolysis and enzyme treatment.
Studies using 90Y-biotin have been successful[14,22]. Biotin has also been labeled with several chelated radionuclides for cancer therapy such as 99mTc, 188Re, 166Ho, and 211At[23-26]. 153Sm is a radiolanthanide, which has not yet been widely used, but possesses nuclear characteristics suitable for RIT. It can be produced in reactors by enriched samarium (152Sm) through the (n, γ) reaction. This enables the production of 153Sm at low cost. As far as we know, studies of labeling antibodies and biotin with 153Sm are very few[27]. It has been recognized that the cation 153Sm3+ has good chelating capabilities with polyaminopolycarboxylic acids, such as EDTA or DTPA. In our study, we chose DTPA as the intermediate chelating agent, which can be linked to the antibodies or SA via bicyclic anhydride (cDTPAa). The main purpose of our investigation was to establish the labeling method of McAb and SA as well as biotin with 153Sm and to evaluate the pretargeting RII and RIT in nude mice bearing human colon carcinoma with SA-biotin system labeled with 153Sm. In the three-step procedure, the tumor was clearly visualized at 4 h in γ-imaging and at the same time point tumor blood pool ratio was 5.76, which was significantly higher than that of control groups. In the two-step procedure, a significant accumulation of 153Sm-SA in the tumor was observed only 24 h after injection. The tumor blood ratios at 4 and 24 h were 1.00 and 2.03, respectively. However, the higher radioactive accumulation was also observed in the liver, spleen, and kidney. This deposition may result from complex formation of biotinylated antibodies with radiolabeled SA in circulation. In addition, in molecule of SA there exists three-peptide amino acid sequences (Arg-Thy-Asp), which may bind to the surface of many types of cells[28]. The advantages of pretargeting technique lie in that it is safe and simple, biotinylation of antibody and other reagents are easily prepared. Since the clearance of radiolabeled biotin or SA from normal tissue is much more rapid than that of directly radiolabeled antibody because of its small molecular weight, background radioactivity levels are drastically reduced, and the high T/NT ratio can be reached shortly after injection of the radiolabel[3,15,29]. Our preliminary studies also showed that compared to directly labeled McAb with 153Sm, multi-step pretargeting could efficiently decrease the blood background levels, elevate the T/NT ratio, shorten the imaging time and improve the quality of imaging. Earlier and better images of tumor can be obtained using these methods, especially the three-step procedure.
RIT has shown disappointing results in bulky, solid tumors, probably due to the low specific accretion of radiolabeled antibody in the tumor target as compared to normal tissues[30]. Tumors have intrinsic characteristics that unfavorably affect localization and intratumoral distribution of McAbs: heterogeneous expression of the antigen, relatively poor tumor vasculature, elevated interstitial pressure and tumor necrosis. Previous investigations have shown that tumor uptake and radiation doses are inversely correlated with tumor size[31,32]. Therefore, RIT may be a viable option, especially for small-volume and minimal residual tumors. 153Sm emits β-rays with intermediate or low energy, which can effectively kill microtumors. In view of these considerations, our RIT experiment was devised to initiate on the 3rd d following tumor inoculation. The results showed that pretargeting RIT and 153Sm-CEA McAb had a strong tumor inhibition effect. The tumor inhibitory rate was 80.67% and 78.44%, 5 wk after therapy. Histopathological evidence also indicated radioactive damage as necrosis of the tumor, which was not seen in the other controls. RIT and pretargeting RIT may be more effective for early stage carcinoma. The reason may be related to lower quantity of tumor cells in early stage, less heterogeneity in tumor antigen expression and greater sensitivity to radiation. In addition, there was, to certain degrees, tumor inhibitory effect in groups of 153Sm-nmIgG and 153Sm-DB2 with a tumor inhibitory rate of 29.78% and 23.99%, respectively, 5 wk after therapy. This may result from unspecific radiation effect. Because 153Sm-nmIgG and 153Sm-DB2 cannot bind specifically with anti-CEA McAb, effective localization in tumor cells in vivo would not take place, thus this unspecific radiation effect is limited.
In three-step pretargeting RIT, 153Sm-DB2 at a single dose of 11.1 MBq/100 μL produced potent tumor-inhibiting effect, and no significant bone marrow toxicity was observed as evidenced by milder decrease of WBC counts. However, in 153Sm-CEA McAb and 153Sm-nmIgG groups, WBC counts significantly decreased, and WBC recovery was also slower than that in three-step pretargeting RIT, indicating that the hemopoietic function of bone marrow might be affected. When 153Sm-CEA McAb and 153Sm-nmIgG enter into blood circulation, its slower blood clearance may result in high background radioactivity levels and cause more radiation-related damage to normal tissues or organs. The rapid clearance of 153Sm-DB2 from blood may drastically reduce background radioactivity levels. Consequently, radiation exposure to normal tissues especially bone marrow is decreased.
In conclusion, pretargeted RIT with 153Sm-DB2 has higher anti-tumor efficacy but lower toxicity than 153Sm-CEA McAb. It may serve as an adjuvant therapy for tumor, particularly for small tumor or micrometastatic disease and residual cancer cells in surgical tumor resection areas. Therefore, it is of good prospect in clinical application.
Science Editor Wang XL, Zhu LH and Guo SY Language Editor Elsevier HK
| 1. | Bian HJ, Chen ZN, Deng JL. Direct technetium-99m labeling of anti-hepatoma monoclonal antibody fragment: a radioimmunoconjugate for hepatocellular carcinoma imaging. World J Gastroenterol. 2000;6:348-352. [PubMed] |
| 2. | Goldenberg DM. Targeted therapy of cancer with radiolabeled antibodies. J Nucl Med. 2002;43:693-713. [PubMed] |
| 3. | Zhang M, Zhang Z, Garmestani K, Schultz J, Axworthy DB, Goldman CK, Brechbiel MW, Carrasquillo JA, Waldmann TA. Pretarget radiotherapy with an anti-CD25 antibody-streptavidin fusion protein was effective in therapy of leukemia/lymphoma xenografts. Proc Natl Acad Sci USA. 2003;100:1891-1895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 74] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Hnatowich DJ, Virzi F, Rusckowski M. Investigations of avidin and biotin for imaging applications. J Nucl Med. 1987;28:1294-1302. [PubMed] |
| 5. | Paganelli G, Malcovati M, Fazio F. Monoclonal antibody pretargetting techniques for tumour localization: the avidin-biotin system. International Workshop on Techniques for Amplification of Tumour Targetting. Nucl Med Commun. 1991;12:211-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 81] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Yao Z, Zhang M, Kobayashi H, Sakahara H, Nakada I, Yamashina J, Konishi J. Improved targeting of radiolabeled streptavidin in tumors pretargeted with biotinylated monoclonal antibodies through an avidin chase. J Nucl Med. 1995;26:837-841. |
| 7. | Paganelli G, Belloni C, Magnani P, Zito F, Pasini A, Sassi I, Meroni M, Mariani M, Vignali M, Siccardi AG. Two-step tumour targeting in ovarian cancer patients using biotinylated monoclonal antibodies and radioactive streptavidin. Eur J Nucl Med. 1992;19:322-329. [RCA] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 61] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Paganelli G, Magnani P, Zito F, Villa E, Sudati F, Lopalco L, Rossetti C, Malcovati M, Chiolerio F, Seccamani E. Three-step monoclonal antibody tumor targeting in carcinoembryonic antigen-positive patients. Cancer Res. 1991;51:5960-5966. [PubMed] |
| 9. | Paganelli G, Stella M, Zito F, Magnani P, De Nardi P, Mangili F, Baratti D, Veglia F, Di Carlo V, Siccardi AG. Radioimmunoguided surgery using iodine-125-labeled biotinylated monoclonal antibodies and cold avidin. J Nucl Med. 1994;35:1970-1975. [PubMed] |
| 10. | Lewis MR, Wang M, Axworthy DB, Theodore LJ, Mallet RW, Fritzberg AR, Welch MJ, Anderson CJ. In vivo evaluation of pretargeted 64Cu for tumor imaging and therapy. J Nucl Med. 2003;44:1284-1292. [PubMed] |
| 11. | Modorati G, Brancato R, Paganelli G, Magnani P, Pavoni R, Fazio F. Immunoscintigraphy with three step monoclonal pretargeting technique in diagnosis of uveal melanoma: preliminary results. Br J Ophthalmol. 1994;78:19-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Boerman OC, van Schaijk FG, Oyen WJ, Corstens FH. Pretargeted radioimmunotherapy of cancer: progress step by step. J Nucl Med. 2003;44:400-411. [PubMed] |
| 13. | Axworthy DB, Reno JM, Hylarides MD, Mallett RW, Theodore LJ, Gustavson LM, Su F, Hobson LJ, Beaumier PL, Fritzberg AR. Cure of human carcinoma xenografts by a single dose of pretargeted yttrium-90 with negligible toxicity. Proc Natl Acad Sci USA. 2000;97:1802-1807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 165] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 14. | Grana C, Chinol M, Robertson C, Mazzetta C, Bartolomei M, De Cicco C, Fiorenza M, Gatti M, Caliceti P, Paganelli G. Pretargeted adjuvant radioimmunotherapy with yttrium-90-biotin in malignant glioma patients: a pilot study. Br J Cancer. 2002;86:207-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 105] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 15. | Paganelli G, Chinol M. Radioimmunotherapy: is avidin-biotin pretargeting the preferred choice among pretargeting methods? Eur J Nucl Med Mol Imaging. 2003;30:773-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Boniface GR, Izard ME, Walker KZ, McKay DR, Sorby PJ, Turner JH, Morris JG. Labeling of monoclonal antibodies with samarium-153 for combined radioimmunoscintigraphy and radioimmunotherapy. J Nucl Med. 1989;30:683-691. [PubMed] |
| 17. | Hnatowich DJ, Childs RL, Lanteigne D, Najafi A. The preparation of DTPA-coupled antibodies radiolabeled with metallic radionuclides: an improved method. J Immunol Methods. 1983;65:147-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 156] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Paganelli G, Pervez S, Siccardi AG, Rowlinson G, Deleide G, Chiolerio F, Malcovati M, Scassellati GA, Epenetos AA. Intraperitoneal radio-localization of tumors pre-targeted by biotinylated monoclonal antibodies. Int J Cancer. 1990;45:1184-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 83] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Magnani P, Paganelli G, Modorati G, Zito F, Songini C, Sudati F, Koch P, Maecke HR, Brancato R, Siccardi AG. Quantitative comparison of direct antibody labeling and tumor pretargeting in uveal melanoma. J Nucl Med. 1996;37:967-971. [PubMed] |
| 20. | Hamblett KJ, Kegley BB, Hamlin DK, Chyan MK, Hyre DE, Press OW, Wilbur DS, Stayton PS. A streptavidin-biotin binding system that minimizes blocking by endogenous biotin. Bioconjug Chem. 2002;13:588-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Kobayashi H, Sakahara H, Hosono M, Yao ZS, Toyama S, Endo K, Konishi J. Improved clearance of radiolabeled biotinylated monoclonal antibody following the infusion of avidin as a "chase" without decreased accumulation in the target tumor. J Nucl Med. 1994;35:1677-1684. [PubMed] |
| 22. | Knox SJ, Goris ML, Tempero M, Weiden PL, Gentner L, Breitz H, Adams GP, Axworthy D, Gaffigan S, Bryan K. Phase II trial of yttrium-90-DOTA-biotin pretargeted by NR-LU-10 antibody/streptavidin in patients with metastatic colon cancer. Clin Cancer Res. 2000;6:406-414. [PubMed] |
| 23. | Correa-Gonzalez L, Arteaga de Murphy C, Ferro-Flores G, Pedraza-Lopez M, Murphy-Stack E, Mino-Leon D, Perez-Villasenor G, Diaz-Torres Y, Munoz-Olvera R. Uptake of 153Sm-DTPA-bis-biotin and 99mTc-DTPA-bis-biotin in rat as-30D-hepatoma cells. Nucl Med Biol. 2003;30:135-140. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Lindegren S, Karlsson B, Jacobsson L, Andersson H, Hultborn R, Skarnemark G. (211)At-labeled and biotinylated effector molecules for pretargeted radioimmunotherapy using poly-L- and poly-D-Lysine as multicarriers. Clin Cancer Res. 2003;9:3873S-3879S. [PubMed] |
| 25. | Ferro-Flores G, Arteaga de Murphy C, Pedraza-López M, Monroy-Guzmán F, Meléndez-Alafort L, Tendilla JI, Jiménez-Varela R. Labeling of biotin with [166Dy]Dy/166Ho as a stable in vivo generator system. Int J Pharm. 2003;255:129-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Ferro-Flores G, Pimentel-González G, González-Zavala MA, Arteaga de Murphy C, Meléndez-Alafort L, Tendilla JI, Croft BY. Preparation, biodistribution, and dosimetry of 188Re-labeled MoAb ior cea1 and its F(ab')2 fragments by avidin-biotin strategy. Nucl Med Biol. 1999;26:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Kraeber-Bodéré F, Mishra A, Thédrez P, Faivre-Chauvet A, Bardiès M, Imai S, Le Boterff J, Chatal JF. Pharmacokinetics and biodistribution of samarium-153-labelled OC125 antibody coupled to CITCDTPA in a xenograft model of ovarian cancer. Eur J Nucl Med. 1996;23:560-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Rosebrough SF, Hartley DF. Biochemical modification of streptavidin and avidin: in vitro and in vivo analysis. J Nucl Med. 1996;37:1380-1384. [PubMed] |
| 29. | Kassis AI, Jones PL, Matalka KZ, Adelstein SJ. Antibody-dependent signal amplification in tumor xenografts after pretreatment with biotinylated monoclonal antibody and avidin or streptavidin. J Nucl Med. 1996;37:343-352. [PubMed] |
| 30. | Behr TM, Goldenberg DM, Becker WS. Radioimmunotherapy of solid tumors: a review "of mice and men". Hybridoma. 1997;16:101-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Behr TM, Sharkey RM, Juweid ME, Dunn RM, Ying Z, Zhang CH, Siegel JA, Goldenberg DM. Variables influencing tumor dosimetry in radioimmunotherapy of CEA-expressing cancers with anti-CEA and antimucin monoclonal antibodies. J Nucl Med. 1997;38:409-418. [PubMed] |
| 32. | Behr TM, Liersch T, Greiner-Bechert L, Griesinger F, Béhé M, Markus PM, Gratz S, Angerstein C, Brittinger G, Becker H. Radioimmunotherapy of small-volume disease of metastatic colorectal cancer. Cancer. 2002;94:1373-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |