Published online Oct 28, 2005. doi: 10.3748/wjg.v11.i40.6281
Revised: December 1, 2004
Accepted: December 3, 2004
Published online: October 28, 2005
AIM: To investigate the cyclooxygenase-2 (COX-2) expression level in human HepG2, Bel-7402 and SMMC-7721 hepatoma cell lines and the molecular mechanism of COX-2 selective inhibitor celecoxib-induced cell growth inhibition and cell apoptosis.
METHODS: Hepatoma cells were cultured and treated with celecoxib. Cell in situ hybridization (ISH) and immunocytochemistry were used to detect COX-2 mRNA and protein expression. Proliferating cell nuclear antigen and phosphorylated Akt were also detected by immunocytochemistry assay. Cell growth rates were assessed by 3-(4, 5-dimethylthiazol-2-yl-2, 5-diphenylte-trazolium (MTT) bromide colorimetric assay. Celecoxib-induced cell apoptosis was measured by terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) and flow cytometry (FCM). The phosphorylated Akt and activated fragments of caspase-9, caspase-3 were examined by Western blotting analysis.
RESULTS: Increased COX-2 mRNA and protein expression were detected in all three hepatoma cell lines. Celecoxib could significantly inhibit cell growth and the inhibitory effect was in a dose- and time-dependent manner evidenced by MTT assays and morphological changes. The apoptotic index measured by TUNEL increased correspondingly with the increased concentration of celecoxib and the reaction time. With 50 μmol/L celecoxib treatment for 24 h, the apoptotic index of HepG2, BEL-7402 and SMMC-7721 cells was 25.01±3.08%, 26.40±3.05%, and 30.60±2.89%, respectively. Western blotting analysis showed remarkable activation of caspase-9, caspase-3 and dephosphorylation of Akt (Thr308). Immunocytochemistry also showed the reduction of PCNA expression and phosphorylation Akt (Thr308) after treatment with celecoxib.
CONCLUSION: COX-2 mRNA and protein overexpression in HepG2, Bel-7402 and SMMC-7721 cell lines correlate with the increased cell growth rate. Celecoxib can inhibit proliferation and induce apoptosis of hepatoma cell strains in a dose- and time-dependent manner.
- Citation: Liu NB, Peng T, Pan C, Yao YY, Shen B, Leng J. Overexpression of cyclooxygenase-2 in human HepG2, Bel-7402 and SMMC-7721 hepatoma cell lines and mechanism of cyclooxygenase-2 selective inhibitor celecoxib-induced cell growth inhibition and apoptosis. World J Gastroenterol 2005; 11(40): 6281-6287
- URL: https://www.wjgnet.com/1007-9327/full/v11/i40/6281.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i40.6281
Cyclooxygenase (COX), known as a PGs synthase, catalyzes the metabolism of arachidonic acid to PGs and thromboxanes, and has three isoforms: COX-1, COX-2 and COX-3[1]. COX-1 is a constitutively expressed enzyme found in most tissues and remains unaltered in cancer. COX-2 is expressed at sites of inflammation, which has led to the speculation that its inhibition could provide all the benefits of current non-steroidal anti-inflammatory drugs (NSAIDs) but without their major side effects on the gastrointestinal system[2,3] (which are due to the inhibition of COX-1). New NSAIDs, which are selective inhibitors of COX-2, can exert therapeutic efficacy without toxic effects due to the inhibition of COX-1. Celecoxib, a selective COX-2 inhibitor approved by US FDA, has been shown preclinically and clinically to have efficacy comparable to that of NSAIDs on relief of pain and inflammation in osteoarthritis.
Recently, COX-2 has been suggested to be associated with carcinogenesis and high expression of COX-2 has been found in many cancers[4-6] such as colorectal cancer, prostatic cancer, breast cancer and gastric cancer. Although selective COX-2 inhibitors could reduce the growth of various cancer cell lines[7-9] and display anti-tumor effect by sensitizing cancer cells to apoptosis[10-12], it is unknown whether COX-2 contributes to the malignant growth and whether inhibition of COX-2 function modifies the malignant potential of tumors.
In hepatocarcinoma (HCC), the expression pattern of COX-2 protein is well correlated with the differentiation grade, suggesting that abnormal COX-2 expression plays an important role in hepatocarcinogenesis[13]. However, the exact anti-tumor mechanisms are not well known. Leng et al[8] have proved that COX-2 could promote HCC cell growth and selective COX-2 inhibitor celecoxib could mediate cell apoptosis in HepG2 and Hep3B cell lines. In this article, we investigate the expression pattern of COX-2 on HepG2 and two other human HCC cell lines, BEL-7402 and SMMC-7721 and evaluate the efficacy of selective COX-2 inhibitor celecoxib in order to understand the potential role of COX-2 in promoting HCC cell growth and to provide theoretical evidence for searching a new agent for the treatment of HCC.
Human HepG2, BEL-7402 and SMMC-7721 hepatoma cell lines were obtained from The Committee of Type Culture Collection of Chinese Academy of Sciences (Shanghai, China). RMPI1640, DMEM and MEM culture medium were purchased from Gibco BRL. Fetal bovine serum (FBS) was provided by Sijiqing Biological Engineering Material (Hangzhou, China). Rabbit anti-COX-2 was from Cayman. Mouse anti-caspase3 was from Boster Biological Technology (Wuhan, China). Rabbit anti-caspase9, anti-actin, rabbit anti-phospho-Akt/PKB (Thr308), sheep anti-rabbit, sheep anti-mouse secondary antibody, Western blotting luminol reagent were purchased from Santa Cruz. In situ cell death detection kit (TUNEL) and sensitive in situ hybridization detection kit were purchased from Roche. Rainbow molecular weight markers were from Amersham. 3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) was provided by Amersco. Celecoxib was provided by Scenry Chemical (Hefei, China).
SMMC-7721 cell line was cultured in DMEM supplemented with 100 mL/L heat-inactivated FBS and 100 U/mL penicillin/streptomycin. Bel-7402 cell line was cultured in RPMI 1640 supplemented with 100 mL/L heat-inactivated FBS and 100 U/mL penicillin/streptomycin. HepG2 cell line was cultured in MEM supplemented with 100 mL/L heat-inactivated FBS, 100 U/mL penicillin/streptomycin and 1% nonessential amino acid. All cells were grown in 50 mL/L CO2 humidified atmosphere at 37 °C.
Celecoxib-treated cells and control were digested by trypsin, washed with phosphate-buffered saline (PBS) and centrifuged at 1 500 r/min for 5 min. After the cells were mixed with glycerine and egg white, the mixtures were fixed in alcohol for 30 min. Then the mass was embedded and fabricated to paraffin section with H&E staining. Morphological changes were observed under light and inverted microscopes.
The digoxigenin-labeled COX-2 oligonucleotide probe for ISH was prepared with the sequence 5’-TGATATCA-TCTAGTCCGGAGCGGGAAG-3’(TaKaRa Bio). ISH was performed according to the instructions of the enhanced sensitive ISH detection kit. Briefly, the sections were deparaffinized with xylene, dehydrated with graded ethanol and embryos were incubated in 1 mL of 20 µg/mL proteinase K for 20 min. Re-fix embryos were incubated for 10 min in 4% formaldehyde. Then all were removed and 20 µL pre-hybridization solution was added for 2 h. Solution was removed and replaced with 20 µL hybridization solution to hybridize overnight at 37 °C. After these, embryos were washed twice in 2× SSC and 0.01× at 37 °C SSC for 5 min each, replaced with anti-digoxygenin-alkaline phosphatase antibody and 40 µL AP-coupled antibodies at 37 °C for 1 h. The last wash was replaced with AP reaction buffer and the reaction was terminated when it became dark.
Celecoxib-treated three cell lines and control were lysed by 4 g/L trypsin containing 0.2 g/L EDTA, then collected after being washed twice with cold PBS. Total protein extract from cells was prepared using cell lysis buffer [50 mmol/L Tris-HCL (pH 8.0), 150 mmol/L NaCl, 1%Triton X-100, 100 μg/mL PMSF, 1 μg/mL aprotinin]. The extract (40 ug) was electrotransferred to nitrocellulose membrane. The membranes were blocked with 5% nonfat dry milk in TBST (10 mmol/L Tris-HCL, pH 8.0, 100 mmol/L NaCl, and 0.05% Tween-20) for 1 h at room temperature and then incubated with appropriate primary antibody overnight at 4 °C, followed by incubation with horseradish peroxidase-conjugated secondary antibody at 1:2 000 dilution for 1 h at room temperature. The polyclonal anti-COX-2 and anti-actin were used at 1:1 000 dilution. The polyclonal anti-caspase-3, anti-caspase-9, anti-phospho-Akt/PKB were used at 1:400 dilution. The immunoblots were visualized by enhanced chemiluminescence.
Briefly, the sections were subjected to immunostaining using an ultrasensitive streptavidin杙eroxidase technique (DAKO). Endogenous peroxidases were blocked by incubating with 5 mL/L H2O2 for 30 min at room temperature. The sections were subsequently treated for 10 min with Triton X-100, and incubated for 30 min at 37 °C with normal non-immune serum before overnight incubation at 4 °C with specific antibody at a dilution of 1:200. The sections were then treated with biotin-conjugated second antibody before streptavidin-peroxidase was added. For color reaction, diaminobenzidine (DAB) was used. If the positive signals were present, the cytoplasm or the nuclei were stained brown. For negative control, the antibody was replaced by PBS.
The number of viable cells was determined by MTT assays. Each cell sample was plated at a density of 10 000 cells/well in 96-well cluster dishes before treatment. On the same day, the medium was changed and celecoxib was added in concentrations and analyzed at indicated time points. After addition of MTT solution (10/100 µL medium) in each well, the cells were incubated at 37 °C for 4 h, and then 100 µL DMSO was added to dissolve the dark blue crystals. The absorbance was measured in an ELISA plate reader with a test wavelength of 570 nm.
Celecoxib-treated cells were fixed with 700 mL/L alcohol for 15 min at 4 °C, and then stained with propidium iodide (PI, Sigma, USA). The red fluorescence of DNA-bound PI in individual cells was measured at 488 nm with a FACSCalibur (Becton Dickinson, USA) and the results were analyzed using CELL QuestV1.22 software.
The apoptotic cells were identified by the terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay according to the manufacturer’s instructions. The cells were counterstained with royal blue. In each experiment, 400 cells were examined using four high-power fields each (magnification×400). The experiments were performed at least twice independently.
The results of TUNEL and immunocytochemistry were analyzed in a blinded fashion by image analysis system (RXA2, Leica) using Qwin software. The apoptosis index (AI) was determined by observing more than 400 nuclei for each sample.
Statistical significance was assessed by the nonparametric ANOVA test with SPSS 10.0 software and P<0.05 was considered statistically significant.
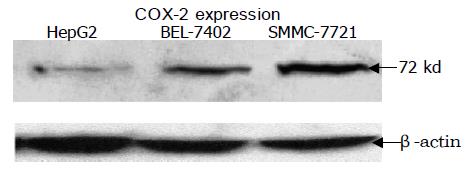
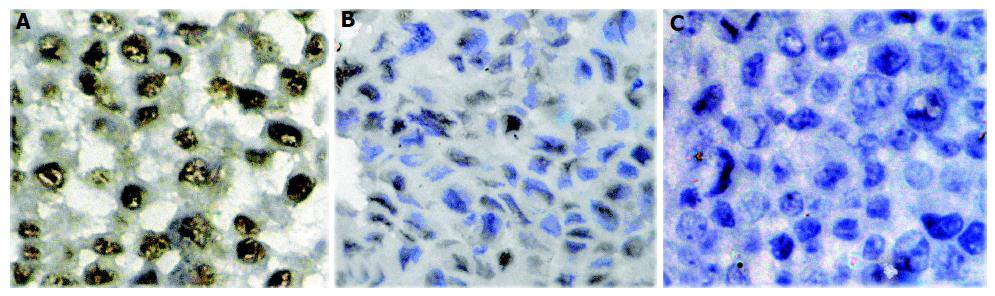
ISH, Western blotting and immunocytochemical staining were performed on HepG2, Bel-7402 and SMMC-7721 cell lines. Significant mRNA and protein of COX-2 were detected in all test cell lines. A representative Western blotting showed that the COX-2 expression level of the HCC cell lines in sequence was SMC-7721>BEL-7402>HepG2 (Figure 1). COX-2 protein was located in the cytoplasm of the cells shown by immunocytochemical staining. No staining was observed in the negative control slide.
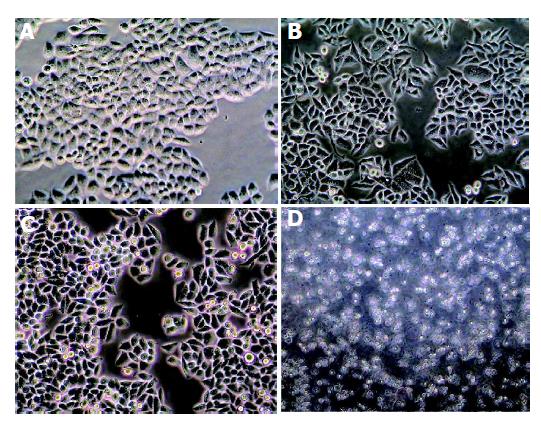
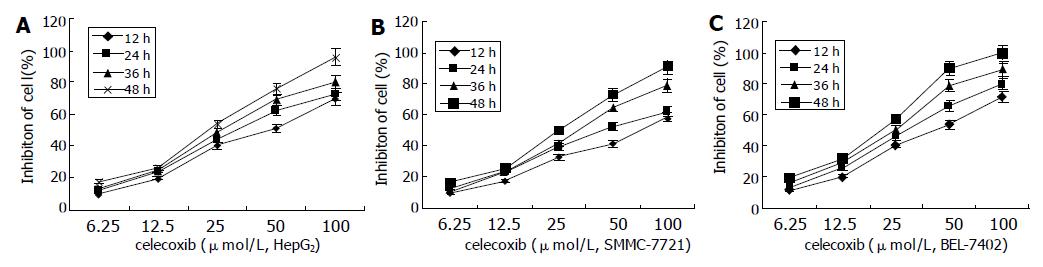
The effects of celecoxib on the cell viability were determined by MTT assay. Cells became sparse, round and detached from the dishes when treated with 6.25, 12.50, 25.00, 50.00, and 100.00 μmol/L concentrations of celecoxib for 24 h (Figure 2), and resulted in 11.9%, 23.9%, 43.8%, 62.4% and 72.7% cell growth reduction, respectively, in HepG2 cells (Figure 3).
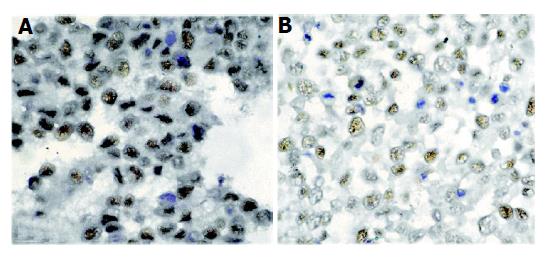
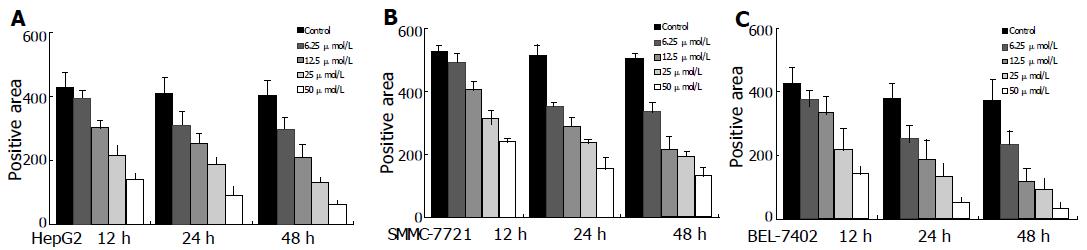
The effects of celecoxib on PCNA expression in cultured HCC cells were assessed by immunocytochemical staining. PCNA-positive cells were more abundant in control cells than in celecoxib-treated cells (Figure 4).
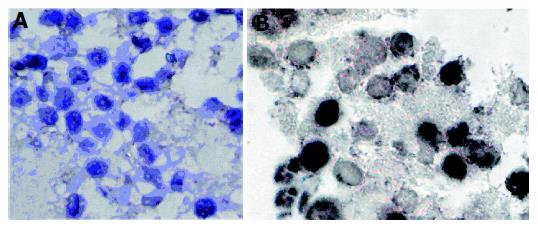
Morphologic changes were evident in the three cell lines after treatment with celecoxib for 24 h. Progressive cytoplasmic shrinkage and nuclear condensation (the typical morphologic signs of apoptosis under light microscope) could be observed. To further make sure of these apoptotic morphologic changes, TUNEL staining was performed. As shown in Figure 5, celecoxib induced HepG2 cell apoptosis in a dose-dependent manner, the percentage of TUNEL-positive cells increased from 2.80±0.84% to 25.01±3.08% after treatment with 6.25 or 50 μmol/L celecoxib for 24 h, respectively. Celecoxib-treated BEL-7402 and SMMC-7721 cell lines showed the same results.
Results of FCM also showed apoptosis induced by celecoxib in SMMC-7721 cell lines. A subdiploid (subG1) peak was observed after being treated with 6.25, 12.50, 25.00 and 50.00 μmol/L celecoxib for 24 h, and the apoptosis rate was 4.84%, 16.10%, 22.80% and 33.80%, respectively, compared to 0.81% in normal control (Table 1).
| Cell line | Control | 6.25 | 12.5 | 25 | 50 |
| BEL- 7402 | 0.60±0.89 | 3.40±1.14 | 7.60±2.41 | 15.20±3.49 | 26.40±3.05 |
| SMMC-7721 | 1.20±0.84 | 4.20±1.48 | 9.80±2.59 | 19.60±3.36 | 30.60±2.89 |
| HepG2 | 1.00±1.00 | 2.80±0.84a | 5.40±1.52 | 15.00±1.58 | 25.01±3.08 |
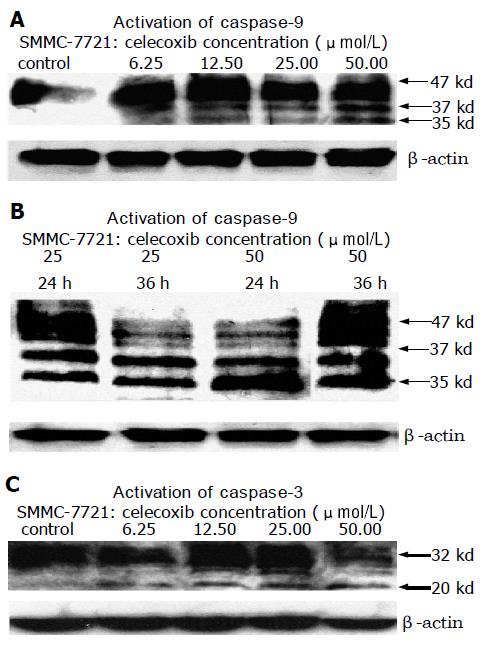
Western blotting analysis revealed that celecoxib induced activation of caspase-9 and caspase-3 (Figure 9). Activation of caspase-3 was reflected by a decrease in the 32-kd precursor and a concomitant increase in the 20-kd cleaved band. Activation of caspase-9 was reflected by conversion of the 47-kd precursor to the active 35- and 37-ku proteolytic cleavage products. The activation level of caspase-9 and caspase-3 was in a dose- and time-dependent manner to the celecoxib treatment (Figure 6).
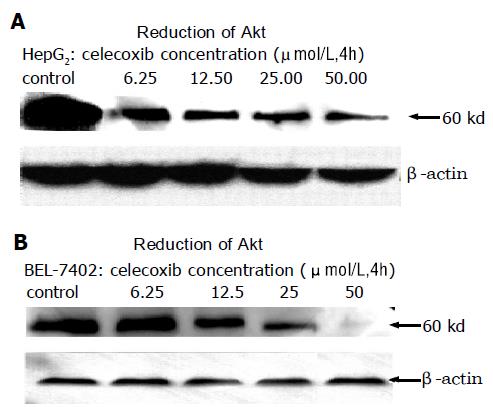
To evaluate the effect of celecoxib on Akt/PKB activity, HepG2, BEL-7402 and SMMC-7721 cells were treated with celecoxib for 4, 12 or 24 h, and phosphorylation at Thr308 was examined by specific phospho-Akt antibody. Western blotting analysis showed that celecoxib significantly reduced Akt/PKB phosphorylation (Figure 7). Immunocy-tochemistry assay also displayed dephosphorylation of Akt Thr308 (Figure 8) in a dose- and time-dependent manner when analyzed with Leica-Qwin computer image analysis system. The results are shown in Figure 9.
COX-2, an isoform of cyclooxygenase, is inducible by a variety of factors including cytokines, growth factors, and tumor promoters[2,3]. It was reported that COX-2 expression increases in many human cancers including HCC[13]. The mechanism leading to COX-2 overexpression in HCC is still unknown. It was demonstrated that overexpression of COX-2 increases cell survival by inhibiting cell apoptosis and stimulating angiogenesis, and enhancing cellular adhesion to the matrix proteins[14,15]. In this article, we have observed a high expression of COX-2 mRNA and protein in human HepG2, Bel-7402 and SMMC-7721 hepatoma cell lines. These results suggest that studies are warranted to determine the role of COX-2 in hepatocarcinogenesis. Furthermore, we have shown that celecoxib, a selective COX-2 inhibitor, could strongly suppress cell proliferation.
Morphologic changes and results obtained by MTT assay support the suggestion that celecoxib down-regulates cell growth activity. It was reported that COX-2 inhibitors may have antineoplastic effects during the early stages of hepatocarcinogenesis[16].
PCNA is an essential protein found in proliferating eukaryotic cells, and plays a crucial role in DNA replication, repair, and control of cell proliferation[17,18]. This protein is involved in the synthesis of both leading and lagging DNA strands, providing an anchorage site and increasing the processivity of DNA polδ and DNA polε, which is the basis of PCNA serving as a DNA synthesis marker for evaluating cell proliferation[19,20]. Therefore, overexpression of COX-2 may be mitogenic in HCC cells through up-regulation of PCNA expression. Selective COX-2 inhibitor celecoxib can block the activity of COX-2 and down-regulate PCNA expression, thus causing cell growth inhibition.
Apoptosis plays a central role in tumor development and lack or failure of apoptosis leads to the development of many tumors including hepatocarcinoma[21,22]. Thus, induction of apoptosis of tumor cells is an effective approach to delay the tumor progress. In this study, we found that celecoxib could induce the characteristic features of apoptosis including morphological changes, TUNEL-positivity, FCM and caspase-9/3 activation of the three tested HCC cell lines.
There are at least two extrinsic and intrinsic broad pathways that lead to apoptosis[23-25]. The “extrinsic” pathway begins with the binding of Fas ligand (FasL or CD95L) to the Fas receptor (CD95) and results in recruiting FADD and pro-caspase-8 to the complex. Concentration of pro-caspase-8 results in its autocatalysis and activation. Activated caspase-8 cleaves pro-caspase-3, which then undergoes autocatalysis to form active caspase-3, a principle effector caspase of apoptosis. The “intrinsic” apoptosis pathway always begins with mitochondrial damage and results in release of cytochrome C from damaged mitochondria. In cytosol or on the surface of mitochondria, cytochrome C is bound to the protein Apaf-1 (apoptotic protease activating factor) which then activates an initiator caspase, caspase-9, then activates caspase-3[26,27]. Caspase families play an important role in apoptosis signaling pathway. They are present in the cytoplasm in normal condition as inactive pro-enzymes, most of which are activated by proteolytic cleavage when undergoing apoptosis[28,29]. Both caspase-8 and caspase-9 can activate the effector caspase, caspase-3, by proteolytic cleavage and subsequent processes result in the nuclear DNA fragmentation and formation of apoptotic bodies, indicating that activation of caspase-3 is a central event in the process of apoptosis.
In this article, Western blotting analysis revealed that celecoxib induced the activation of caspase-9 and caspase-3. Activation of caspase-9 is reflected by conversion of the 47-kd inactive precursor to the active 35-kd and 37-kd proteolytic cleavage products. Activation of caspase-3 is indicated by a decrease of the 32-kd precursor accompanied with a concomitant increase in the 20-kd cleaved band. Leng et al[8], reported that celecoxib-treated HepG2 cells significantly release cytochrome C in the cytosol. Thus we propose the hypothesis that celecoxib allows the release of cytochrome C from mitochondria and activates caspase-9 and caspase-3 in sequence and finally induces apoptosis of HCC cells.
Our works have also shown that celecoxib down-regulates the phosphorylation of Akt/PKB at Thr308 in a dose- and time-dependent manner. Akt/PKB[30,31], the major downstream effector of PI3-kinase, is a Ser/Thr protein kinase that exerts a crucial role in the regulation of several cellular signaling pathways. Akt/PKB is a regulator of cell survival and apoptosis, and its activation can protect a variety of cells against apoptosis. Akt/PKB is phosphorylated at two regulatory sites, Thr308 and Ser473, essential for its activation. Activated Akt/PKB can phosphorylate BAD, IκB kinase, glycogen synthase kinase-3β, and forkhead transcription factors[32,33], leading to their inactivation and cell survival. Interestingly, it was reported that caspase-9 activity is regulated by phosphorylation[34]. Akt phosphorylates pro-caspase-9 at Ser196 and inhibits proteolytic processing of pro-caspase-9.
Celecoxib blocks the activation of Akt, thereby attenuating the activity of a major anti-apoptotic pathway and inducing cell apoptosis. It remains unclear how celecoxib affects Akt phosphorylation because it does not affect PI3-kinase activity directly[35]. Other potential consequences of COX-2 inhibition, such as modulation of the RAS-signaling pathway, p53 expression, and other members of the BCL-2 family, such as MCL-1[36,37], activation of the sphingomyelin-ceramide pathway, and interference with the NF-κB[38] need to be investigated in future.
In conclusion, COX-2 is overexpressed in human HepG2, BEL-7402 and SMMC-7721 hepatoma cells and may play a key role in tumor cell proliferation and carcinogenesis. Selective COX-2 inhibitor celecoxib can suppress the growth of HCC cells by inhibiting cell growth and inducing cell apoptosis. The mechanism of apoptosis induced by celecoxib is due to activation of the caspase cascade, which is correlated with phosphorylation of Akt/PKB. These results suggest that selective COX-2 inhibitor offers potential as an effective chemotherapeutic and chemopreventive strategy against human liver cancer.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Chandrasekharan NV, Dai H, Roos KL, Evanson NK, Tomsik J, Elton TS, Simmons DL. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression. Proc Natl Acad Sci USA. 2002;99:13926-13931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1314] [Cited by in RCA: 1199] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 2. | Zhang F, Warskulat U, Wettstein M, Schreiber R, Henninger HP, Decker K, Häussinger D. Hyperosmolarity stimulates prostaglandin synthesis and cyclooxygenase-2 expression in activated rat liver macrophages. Biochem J. 1995;312:135-143. [PubMed] |
| 3. | Feng L, Xia Y, Yoshimura T, Wilson CB. Modulation of neutrophil influx in glomerulonephritis in the rat with anti-macrophage inflammatory protein-2 (MIP-2) antibody. J Clin Invest. 1995;95:1009-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 127] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 4. | Hussain T, Gupta S, Mukhtar H. Cyclooxygenase-2 and prostate carcinogenesis. Cancer Lett. 2003;191:125-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 164] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 5. | Peng JP, Su CY, Chang HC, Chai CY, Hung WC. Overexpression of cyclo-oxygenase 2 in squamous cell carcinoma of the hypopharynx. Hum Pathol. 2002;33:100-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Sheng H, Shao J, Morrow JD, Beauchamp RD, DuBois RN. Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res. 1998;58:362-366. [PubMed] |
| 7. | Vogiagis D, Brown W, Glare EM, O払rien PE. Rat colorectal tumours treated with a range of non-steroidal anti-inflammatory drugs show altered cyclooxygenase-2 and cyclooxygenase-1 splice variant mRNA expression levels. Carcinogenesis. 2001;22:869-874. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Leng J, Han C, Demetris AJ, Michalopoulos GK, Wu T. Cyclooxygenase-2 promotes hepatocellular carcinoma cell growth through Akt activation: evidence for Akt inhibition in celecoxib-induced apoptosis. Hepatology. 2003;38:756-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 214] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 9. | Kinoshita T, Takahashi Y, Sakashita T, Inoue H, Tanabe T, Yoshimoto T. Growth stimulation and induction of epidermal growth factor receptor by overexpression of cyclooxygenases 1 and 2 in human colon carcinoma cells. Biochem Biophys Acta. 1999;1438:120-130. [RCA] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 80] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Johnsen JI, Lindskog M, Ponthan F, Pettersen I, Elfman L, Orrego A, Sveinbjörnsson B, Kogner P. Cyclooxygenase-2 is expressed in neuroblastoma, and nonsteroidal anti-inflammatory drugs induce apoptosis and inhibit tumor growth in vivo. Cancer Res. 2004;64:7210-7215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 89] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 11. | Jacoby RF, Seibert K, Cole CE, Kelloff G, Lubet RA. The cyclooxygenase-2 inhibitor celecoxib is a potent preventive and therapeutic agent in the min mouse model of adenomatous polyposis. Cancer Res. 2000;60:5040-5044. [PubMed] |
| 12. | Kanayama N, Terao T. Plasma fibronectin receptor levels during pregnancy complicated by preeclampsia and abruptio placentae. Gynecol Obstet Invest. 1992;33:147-152. [PubMed] |
| 13. | Bae SH, Jung ES, Park YM, Kim BS, Kim BK, Kim DG, Ryu WS. Expression of cyclooxygenase-2 (COX-2) in hepatocellular carcinoma and growth inhibition of hepatoma cell lines by a COX-2 inhibitor, NS-398. Clin Cancer Res. 2001;7:1410-1418. [PubMed] |
| 14. | Masunaga R, Kohno H, Dhar DK, Ohno S, Shibakita M, Kinugasa S, Yoshimura H, Tachibana M, Kubota H, Nagasue N. Cyclooxygenase-2 expression correlates with tumor neovascularization and prognosis in human colorectal carcinoma patients. Clin Cancer Res. 2000;6:4064-4068. [PubMed] |
| 15. | Fujiwaki R, Iida K, Kanasaki H, Ozaki T, Hata K, Miyazaki K. Cyclooxygenase-2 expression in endometrial cancer: correlation with microvessel count and expression of vascular endothelial growth factor and thymidine phosphorylase. Hum Pathol. 2002;33:213-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 84] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Denda A, Kitayama W, Murata A, Kishida H, Sasaki Y, Kusuoka O, Tsujiuchi T, Tsutsumi M, Nakae D, Takagi H. Increased expression of cyclooxygenase-2 protein during rat hepatocarcinogenesis caused by a choline-deficient, L-amino acid-defined diet and chemopreventive efficacy of a specific inhibitor, nimesulide. Carcinogenesis. 2002;23:245-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Zheleva DI, Zhelev NZ, Fischer PM, Duff SV, Warbrick E, Blake DG, Lane DP. A quantitative study of the in vitro binding of the C-terminal domain of p21 to PCNA: affinity, stoichiometry, and thermodynamics. Biochemistry. 2000;39:7388-7397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Kelman Z. PCNA: structure, functions and interactions. Oncogene. 1997;14:629-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 630] [Cited by in RCA: 660] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 19. | Kurki P, Vanderlaan M, Dolbeare F, Gray J, Tan EM. Expression of proliferating cell nuclear antigen (PCNA)/cyclin during the cell cycle. Exp Cell Res. 1986;166:209-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 382] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 20. | Robbins BA, de la Vega D, Ogata K, Tan EM, Nakamura RM. Immunohistochemical detection of proliferating cell nuclear antigen in solid human malignancies. Arch Pathol Lab Med. 1987;111:841-845. [PubMed] |
| 21. | Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239-257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9960] [Cited by in RCA: 10001] [Article Influence: 188.7] [Reference Citation Analysis (0)] |
| 22. | Evan G, Littlewood T. A matter of life and cell death. Science. 1998;281:1317-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1079] [Cited by in RCA: 1046] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 23. | Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng TI, Jones DP, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3677] [Cited by in RCA: 3688] [Article Influence: 131.7] [Reference Citation Analysis (0)] |
| 24. | Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5324] [Cited by in RCA: 5479] [Article Influence: 195.7] [Reference Citation Analysis (0)] |
| 25. | Bossy-Wetzel E, Newmeyer DD, Green DR. Mitochondrial cytochrome c release in apoptosis occurs upstream of DEVD-specific caspase activation and independently of mitochondrial transmembrane depolarization. EMBO J. 1998;17:37-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 931] [Cited by in RCA: 958] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 26. | Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3727] [Cited by in RCA: 3768] [Article Influence: 129.9] [Reference Citation Analysis (0)] |
| 27. | Zou H, Henzel WJ, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997;90:405-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2262] [Cited by in RCA: 2263] [Article Influence: 80.8] [Reference Citation Analysis (0)] |
| 28. | Sakai T, Liu L, Teng X, Mukai-Sakai R, Shimada H, Kaji R, Mitani T, Matsumoto M, Toida K, Ishimura K. Nucling recruits Apaf-1/pro-caspase-9 complex for the induction of stress-induced apoptosis. J Biol Chem. 2004;279:41131-41140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Arnoult D, Gaume B, Karbowski M, Sharpe JC, Cecconi F, Youle RJ. Mitochondrial release of AIF and EndoG requires caspase activation downstream of Bax/Bak-mediated permeabilization. EMBO J. 2003;22:4385-4399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 338] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 30. | Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, Segal RA, Kaplan DR, Greenberg ME. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1833] [Cited by in RCA: 1928] [Article Influence: 68.9] [Reference Citation Analysis (0)] |
| 31. | Franke TF, Kaplan DR, Cantley LC. PI3K: downstream AKTion blocks apoptosis. Cell. 1997;88:435-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1304] [Cited by in RCA: 1333] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 32. | Fang X, Yu S, Eder A, Mao M, Bast RC, Boyd D, Mills GB. Regulation of BAD phosphorylation at serine 112 by the Ras-mitogen-activated protein kinase pathway. Oncogene. 1999;18:6635-6640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 215] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 33. | Rena G, Prescott AR, Guo S, Cohen P, Unterman TG. Roles of the forkhead in rhabdomyosarcoma (FKHR) phosphorylation sites in regulating 14-3-3 binding, transactivation and nuclear targetting. Biochem J. 2001;354:605-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 119] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 34. | Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2297] [Cited by in RCA: 2291] [Article Influence: 84.9] [Reference Citation Analysis (0)] |
| 35. | Zhou H, Summers SA, Birnbaum MJ, Pittman RN. Inhibition of Akt kinase by cell-permeable ceramide and its implications for ceramide-induced apoptosis. J Biol Chem. 1998;273:16568-16575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 280] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 36. | Hsu AL, Ching TT, Wang DS, Song X, Rangnekar VM, Chen CS. The cyclooxygenase-2 inhibitor celecoxib induces apoptosis by blocking Akt activation in human prostate cancer cells independently of Bcl-2. J Biol Chem. 2000;275:11397-11403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 513] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 37. | Lin MT, Lee RC, Yang PC, Ho FM, Kuo ML. Cyclooxygenase-2 inducing Mcl-1-dependent survival mechanism in human lung adenocarcinoma CL1.0 cells. Involvement of phosphatidylinositol 3-kinase/Akt pathway. J Biol Chem. 2001;276:48997-49002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 144] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 38. | Lee JY, Ye J, Gao Z, Youn HS, Lee WH, Zhao L, Sizemore N, Hwang DH. Reciprocal modulation of Toll-like receptor-4 signaling pathways involving MyD88 and phosphatidylinositol 3-kinase/AKT by saturated and polyunsaturated fatty acids. J Biol Chem. 2003;278:37041-37051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 401] [Article Influence: 18.2] [Reference Citation Analysis (0)] |