Published online Oct 28, 2005. doi: 10.3748/wjg.v11.i40.6277
Revised: July 12, 2005
Accepted: July 15, 2005
Published online: October 28, 2005
AIM: To evaluate the therapeutic effect and the indication of percutaneous ethanol injection (PEI), radiofrequency ablation (RFA) and their combination in treatment of hepatocellular carcinoma (HCC).
METHODS: Two hundred and fifty-five patients with HCC received treatment of PEI, RFA or their combination. Group1 (< 3 cm in diameter, n=85) was treated with PEI, group2 (< 3 cm in diameter, n=153) with RFA. Group3 (>3 cm in diameter, n=86) was divided into two groups. Group 3a (n=34) was treated with RFA, while group 3b (n=52) was treated with RFA for 2 wk after transcatheter arterial chemoembolization or PEI. Contrast-enhanced sonography was performed for 61 patients before and after RFA. Liver function and serum alpha-fetoprotein (AFP) were measured for all patients. Changes of the lesions on ultrasound and contrast-enhanced CT/MRI were evaluated for assessing the therapeutic responses. The 1-, 2-, 3- and 5-year survival rates were recorded after treatment.
RESULTS: In group 1, the complete necrosis rate of lesions after 1 mo was 77.6% (66/85). The level of AFP declined conspicuously after 1 mo. The 1-, 2-, 3- and 5-year survival rate after treatment was 80.0% (52/65), 60.4% (32/53), 52.5% (21/40) and 33.3% (7/21), respectively. In group 2, the complete necrosis rate of lesions after 1 moh was 92.2% (141/153). The level of AFP decreased conspicuously after 1 mo. The 1-, 2-, 3- and 5-year survival rate after treatment was 94.6% (88/93), 73.2% (52/71), 63.5% (33/52) and 46.4% (13/28), respectively. In group 3a, the complete necrosis rate of lesions after 1 mo was 23.5% (8/34). AFP dropped down to the normal level in only one patient after 1 mo. The 1-, 2- and 3-year survival rate after treatment was 47.6% (10/21), 42.9% (6/14) and 27.3% (3/11), respectively. Only one patient was still alive after 5 years. In group 3b, the complete necrosis rate of lesions after 1 mo was 57.7% (30/52). The level of AFP decreased after 1 mo. The 1-, 2-, 3- and 5-year survival rate after treatment was 68.6% (24/35), 46.2% (12/26), 36.8% (7/19) and 27.3% (3/11), respectively.
CONCLUSION: The therapeutic effect of RFA on small HCC is better than that of PEI. Small HCC is the optimal indication of RFA. For recurrent HCC (diameter>3 cm), the combined treatment of RFA and PEI/ACE should be used.
- Citation: Luo BM, Wen YL, Yang HY, Zhi H, Xiao XY, Ou B, Pan JS, Ma JH. Percutaneous ethanol injection, radiofrequency and their combination in treatment of hepatocellular carcinoma. World J Gastroenterol 2005; 11(40): 6277-6280
- URL: https://www.wjgnet.com/1007-9327/full/v11/i40/6277.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i40.6277
With the advance in surgical techniques, surgical re-section in combination with intervention therapy for hepatocellular carcinoma (HCC) has been developed. This combined treatment has improved the survival rate of HCC patients. However, most HCC patients lose the chance of resection because they are in advanced stage of the disease when they see a doctor. Furthermore, some HCCs are multicenter in origin. All these factors lead to a high recurrence after resection[1-4]. Though patients with recurrent tumor can undergo re-resection, most of them do not endure it because of poor liver function[5] or poor quality of life after surgery. Therefore, non-surgical partial ablative treatments, such as transcatheter arterial chemoembolization (TACE), PEI, RFA and microwave ablation, are commonly used for HCC. These methods have their own advantages and limitations. It is worth discussing how to choose a suitable method according to the different situation of patients to achieve the best therapeutic effect. This study aimed to compare the therapeutic effects of different treatments.
From August 1999 to May 2005, 255 patients with HCC received the treatment of PEI, RFA or their combination. There were 187 men and 68 women. Their age ranged from 31 to 72 years, Two hundred and eleven of the 255 patients had liver cirrhosis due to hepatitis B (n=206) or alcoholic hepatitis (n=5). Their liver function was Child A in 36, Child B in 208 and Child C in 11.
In group 1, 71 patients were treated with PEI. The diameter of the lesions ranged 1.32~2.91 cm (2.21±1.48 cm). In group 2, 118 patients were treated with RFA. The diameter of the lesions ranged 1.07~2.96 cm (2.39±1.57 cm). In group 3, 24 patients were treated with RFA (group 3a). The diameter of the lesions ranged 3.09~6.18 cm (4.86±2.24 cm). The remaining 42 patients underwent RFA after TACE or PEI. The diameter of the lesions ranged 3.56~7.21 cm (5.44±2.87 cm). The level of alanine aminotransferase in each patient before and two weeks after treatment was measured. AFP level and imaging changes on B-mode sonography, color Doppler flow imaging (CDFI) and contrast-enhanced CT/MR were also evaluated. Contrast-enhanced ultrasound (CEUS) was performed in 61 patients before and after RFA. The 1-, 2-, 3- and 5-year survival rates were recorded. If hypervascularity was observed in the lesions by imaging techniques, TACE or PEI was given followed by FRA within one week. On the contrary, if the lesions were hypovascular, RFA was performed. In the patients with partial necrosis of the lesions, RFA or PEI was done again in the remaining part.
The criteria for the therapeutic effect were based on the imagings. Complete necrosis: no enhancement of the lesion on contrast-enhanced CT/MR 1 mo after treatment and no blood flow on CDFI or CEUS with enhanced echo of the whole lesions; partial necrosis: partial enhancement of the lesion on contrast-enhanced CT/MR 1 month after treatment and blood flow signals of the lesion on CDFI or CEUS with partial or no enhancement of the echo in the whole lesion. The 1-, 2-, 3- and 5-year survival rates after treatments were recorded.
A cool tip RF system (Radionics, UAS) was used in the study. The cooling liquid used was 0 °C pure water. The puncture was guided by Esaote Technos MPX (Esaote SpA, Italy) or ALOKA SSD-2000 (Aloka Co., Japan) sonographic unit.
Statistical analysis was done using the SPSS 11.0. Homogeneity test of variance was performed before statistical analysis. Medians were used to describe the data in non-normal distribution. The data in normal distribution were expressed as mean±SD. T-test was used to analyze the difference between the two groups. Paired t-test was used to detect the difference before and after treatment. Analysis of variance and paired Snk-q test were used to detect the differences among the groups. P<0.05 was considered statistically significant.
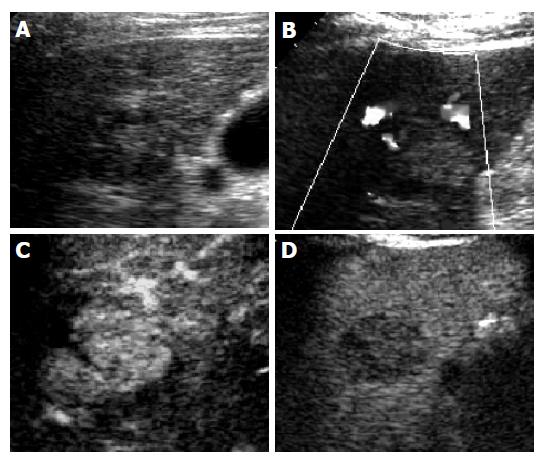
In group 1, a total of 268 times of PEI were performed with guidance of ultrasound in 71 patients with HCC (mean, 3.2 times/lesion). Eleven lesions received PEI once, 15 lesions PEI twice, 19 lesions PEI thrice, 30 lesions PEI 4 times and 10 lesions PEI 5 times. The complete necrosis rate 1 mo after the treatment was 77.6% (66/85). The AFP level before treatment ranged 11.8 - 4102 µg/mL (median, 192.6 µg/mL) and was higher than 25μg/mL in 48 of the patients and dropped below 25 µg/mL 1 mo after the treatment in 31 of them. But the AFP level was even higher than before in 4 patients. The AFP level after treatment ranged 2.8~2313 µg/mL (median, 17.7 µg/mL). The 1-, 2-, 3- and 5-year survival rate after treatment was 80.0% (52/65), 60.4% (32/53), 52.5% (21/40) and 33.3% (7/21), respectively (Figure 1).
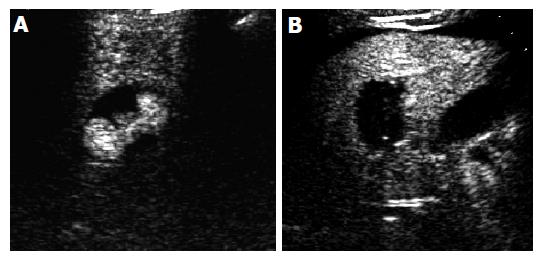
In group 2, 118 patients received RFA 199 times (mean, 1.3 times/lesion). Ninety-five lesions received RFA once, 35 lesions RFA twice and 13 lesions RFA thrice. The complete necrosis rate 1 mo after treatment was 92.2% (141/153). The level of AFP (2.8~31.3 µg/mL, median 18.5 µg/mL) decreased 1 mo after treatment in 74 patients. The 1-, 2-, 3- and 5-year survival rate was 94.6% (88/93), 73.2% (52/71), 63.5% (33/52) and 46.4% (13/28), respectively (Figure 2).
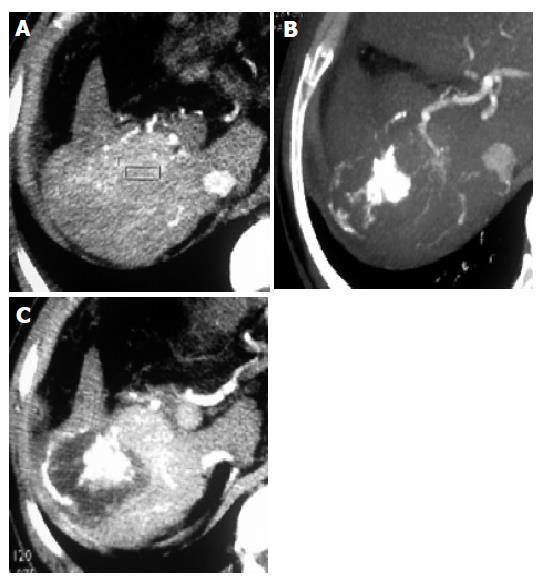
In group 3a, 24 patients received RFA 91 times (mean, 2.7 times/lesion). Four lesions received RFA once, 10 lesions RFA twice, 13 lesions RFA thrice, 7 lesions RFA 4 times. The complete necrosis rate 1 mo after treatment was 23.5% (8/34). Before treatment, the level of AFP ranged 5.8 - 11 246 µg/mL (median, 417.3 µg/mL). One month after treatment, the level of AFP ranged 12.8~13 513 µg/mL (median, 412.2 µg/mL). The level of AFP dropped below 25 µg/mL in only 1 patient. The 1-, 2-, 3-year survival rate was 47.6% (10/21), 42.9% (6/14) and 27.3% (3/11), respectively. Only one patient was alive after 5 years (Figure 3).
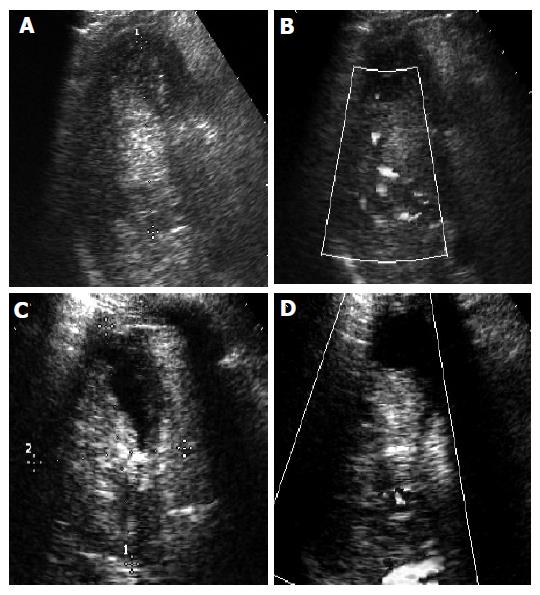
In group 3b, 42 patients received RFA 110 times (mean, 2.1 times/lesion) after TACE or PEI. Fourteen lesions received RFA once, 23 lesions RFA twice, 10 lesions RFA thrice, 5 lesions RFA 4 times. The complete necrosis rate 1 mo after treatment was 57.7% (30/52). Before treatment, the level of AFP ranged 4.7 - 10 247 µg/mL (median, 423.9 µg/mL). One month after treatment, the level of AFP ranged 5.7~8912 µg/mL (median, 17.7 µg/mL). The 1-, 2-, 3- and 5-year survival rate was 68.6% (24/35), 46.2% (12/26), 36.8% (7/19) and 27.3% (3/11), respectively (Figure 4).
There was no significant difference in the levels of ALT before and after RFA. Two weeks after treatment, the level of ALT (73±38 IU) in group 1 was higher than that (32±23 IU) before treatment (P<0.05). There was no significant difference in the level of ALT (36±25 IU) 4 wk after treatment compared to that before treatment (P>0.05).
PEI has become a new interventional method for HCC, and better therapeutic effect has been achieved[6,7]. However, because of the repetitive injection and the inhomogeneous diffusion of ethanol within the tumor, it is difficult to achieve complete necrosis of some large inhomogeneous tumors. In addition, the treatment is often suspended because of unendurable pain. Furthermore, PEI decreases the immune function of patients and leads to severe impairment of liver function because of the damage to peripheral liver tissue caused by diffusion of ethanol. In patients with liver cirrhosis before treatment, the curative dose of ethanol may elicit severe complications such as hemorrhage of the congestive ducts or liver failure[8]. RFA is a local chemotherapy and kills tumor cells more completely with less influence on the peripheral liver tissue. This study showed that the complete necrosis rate of lesions and the 1-, 2-, 3- and 5-year survival rates of patients receiving PEI were significantly lower than those of patients receiving RFA. The lower therapeutic effect, worse complications and severer pain of PEI compared to RFA suggest that it is better to choose RFA for treatment of small HCC.
Furthermore, the complete necrosis rate of lesions in group 2 one month after the treatment was 92.2% (141/153). The level of AFP in this group decreased conspicuously. The 1-, 2-, 3- and 5-year survival rates after treatment (94.6%, 73.2%, 63.5%, and 46.4%, respectively) were significantly higher than those of in groups 3a and 3b, suggesting that the therapeutic effect of RFA is good, which is comparable to that of surgical resection.
However, even RFA has better therapeutic effect of HCC with fewer side effects[9-12], it is difficult to obtain complete coagulation in large HCC. One of the limitations of RFA is the small volume of treatment because of the heat from the pole is quickly decreased when it is transmitted in tumor tissue. When the electrode is used, the largest-treated area is about 3 cm in diameter, resulting in more times of RFA and worse therapeutic effect[13] in large tumors. In this study, even more times of ablation were performed in group 3a, the complete necrosis rate was lower than that in group 2. The residual tumor in the treated area maybe one of the most important reasons.
Many different methods have been used to obtain a larger treated area of RFA. TACE is one of the most commonly used non-surgical methods for HCC. It can acquire good therapeutic effect in enveloped small HCC[14]. But for HCCs without envelope or the envelope has been invaded, even small HCC can not be completely necrotized by TACE[15]. However, TACE can reduce the blood supply for the tumor[16]. In group 3b, TACE was performed before RFA in 23 patients (27 lesions), while PEI was performed before RFA in 19 patients (25 lesions). The complete necrosis rate after 1 mo was 57.7% (30/52) in group 3b, significantly higher than that in group 3a (23.5%, 8/34) (P<0.05). Therefore, when the tumor is larger than 3 cm in diameter, combined treatment of RFA with TACE or PEI is suggested in order to improve the therapeutic effect.
In conclusion, RFA is one of the optimal choices of treatment for small HCC and combined treatment is suggested for larger HCC.
Co-first-authors: Bao-Ming Luo and Yan-Ling Wen
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Poon RT, Fan ST, Ng IO, Lo CM, Liu CL, Wong J. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer. 2000;89:500-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 2. | Tang ZY, Yu YQ, Zhou XD, Ma ZC, Wu ZQ. Progress and prospects in hepatocellular carcinoma surgery. Ann Chir. 1998;52:558-563. [PubMed] |
| 3. | Figueras J, Jaurrieta E, Valls C, Ramos E, Serrano T, Rafecas A, Fabregat J, Torras J. Resection or transplantation for hepatocellular carcinoma in cirrhotic patients: outcomes based on indicated treatment strategy. J Am Coll Surg. 2000;190:580-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 196] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 4. | Belghiti J, Hiramatsu K, Benoist S, Massault P, Sauvanet A, Farges O. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191:38-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 800] [Cited by in RCA: 798] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 5. | Tang ZY. Treatment of hepatocellular carcinoma. Digestion. 1998;59:556-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Livraghi T, Giorgio A, Marin G, Salmi A, de Sio I, Bolondi L, Pompili M, Brunello F, Lazzaroni S, Torzilli G. Hepatocellular carcinoma and cirrhosis in 746 patients: long-term results of percutaneous ethanol injection. Radiology. 1995;197:101-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 603] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 7. | Shiina S, Tagawa K, Niwa Y, Unuma T, Komatsu Y, Yoshiura K, Hamada E, Takahashi M, Shiratori Y, Terano A. Percutaneous ethanol injection therapy for hepatocellular carcinoma: results in 146 patients. AJR Am J Roentgenol. 1993;160:1023-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 251] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 8. | Livraghi T. Percutaneous ethanol injection in the treatment of hepatocellular carcinoma in cirrhosis. Hepatogastroenterology. 2001;48:20-24. [PubMed] |
| 9. | Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma & lt; or =4 cm. Gastroenterology. 2004;127:1714-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 437] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 10. | Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Ierace T, Solbiati L, Gazelle GS. Hepatocellular carcinoma: radio-frequency ablation of medium and large lesions. Radiology. 2000;214:761-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 806] [Cited by in RCA: 740] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 11. | Solbiati L, Goldberg SN, Ierace T, Livraghi T, Meloni F, Dellanoce M, Sironi S, Gazelle GS. Hepatic metastases: percutaneous radio-frequency ablation with cooled-tip electrodes. Radiology. 1997;205:367-373. [PubMed] |
| 12. | Francica G, Marone G. Ultrasound-guided percutaneous treatment of hepatocellular carcinoma by radiofrequency hyperthermia with a 'cooled-tip needle'. A preliminary clinical experience. Eur J Ultrasound. 1999;9:145-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 86] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Rossi S, Garbagnati F, Lencioni R, Allgaier HP, Marchianò A, Fornari F, Quaretti P, Tolla GD, Ambrosi C, Mazzaferro V. Percutaneous radio-frequency thermal ablation of nonresectable hepatocellular carcinoma after occlusion of tumor blood supply. Radiology. 2000;217:119-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 345] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 14. | Choi BI, Kim HC, Han JK, Park JH, Kim YI, Kim ST, Lee HS, Kim CY, Han MC. Therapeutic effect of transcatheter oily chemoembolization therapy for encapsulated nodular hepatocellular carcinoma: CT and pathologic findings. Radiology. 1992;182:709-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 132] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Kuroda C, Sakurai M, Monden M, Marukawa T, Hosoki T, Tokunaga K, Wakasa K, Okamura J, Kozuka T. Limitation of transcatheter arterial chemoembolization using iodized oil for small hepatocellular carcinoma. A study in resected cases. Cancer. 1991;67:81-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 16. | Cheng HY, Shou Y, Wang X, Xu AM, Chen D, Jia YC. Adjustment of lipiodol dose according to tumor blood supply during transcatheter arterial chemoembolization for large hepatocellular carcinoma by multidetector helical CT. World J Gastroenterol. 2004;10:2753-2755. [PubMed] |