Published online Oct 28, 2005. doi: 10.3748/wjg.v11.i40.6258
Revised: October 4, 2004
Accepted: October 7, 2004
Published online: October 28, 2005
AIM: To investigate the reduction of cell viability in human hepatocellular carcinoma (HCC) cell lines induced by inhibition of nuclear factor κB (NFκB).
METHODS: HLE, SKHep1, and HepG2 were incubated and E3330 was used to compare the stimulation of some chemotherapeutic drugs with that of TNF family, Fas ligand, TNFα and TNF-related apoptosis-inducing ligand (TRAIL) at the point of the reduction of cell viability by inhibiting NFκB.
RESULTS: E3330 decreased NFκB levels in HLE cells stimulated by TNF and TRAIL. The cytotoxicity of the combination of TRAIL, TNFα, Fas ligand, and E3330 increased synergistically in a dose-dependent manner compared to either E3330 alone in all HCC cell lines by MTT assay. However, the combination of some chemotherapeutic drugs and E3330 did not decrease the cell viability.
CONCLUSION: Inhibition of NFκB sensitizes human HCC cell lines to TNF-mediated apoptosis including TRAIL, and TRAIL-based tumor therapy might be a powerful potential therapeutic tool in the treatment of human HCC.
- Citation: Saitou Y, Shiraki K, Yamanaka T, Miyashita K, Inoue T, Yamanaka Y, Yamaguchi Y, Enokimura N, Yamamoto N, Itou K, Sugimoto K, Nakano T. Augmentation of tumor necrosis factor family-induced apoptosis by E3330 in human hepatocellular carcinoma cell lines via inhibition of NFκB. World J Gastroenterol 2005; 11(40): 6258-6261
- URL: https://www.wjgnet.com/1007-9327/full/v11/i40/6258.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i40.6258
The process of apoptosis is fundamental in the deve-lopmental and homeostatic maintenance of complex biological systems[1,2]. Dysregulation or failure of normal apoptotic mechanisms contributes to the transformation of cells and provides a growth advantage to cancer cells[3]. TNF family members, such as TNFα and Fas ligand, play an important role in determining cell death or survival in a variety of human cells and transformed cells[2,4]. However, the potential use of systemically administered TNFα and Fas ligand is limited by their acute cytotoxic effects on normal tissues in vivo, thereby limiting their widespread use in the treatment of cancer[5,6]. In contrast, TNF-related apoptosis-inducing ligand (TRAIL) is able to kill a wide spectrum of tumor cells, and appears to be nontoxic to most normal cells[7].
Nuclear factor κB (NFκB) is an essential survival factor in many physiological conditions, such as embryonic liver development and liver regeneration[8]. In liver neoplasm, NFκB plays an important role in the resistance to TNF cytotoxicity and functional pathways including TNF receptor-associated factor 2[9].
Compound E3330 has a therapeutic effect in murine models of endotoxin-mediated hepatitis and in rats with galactosamine-induced hepatitis, presumably as a result of E3330 inhibition of TNFα generation[10,11]. E3330 inhibits NFκB DNA binding, most probably via an interaction with a nuclear factor that activates NFκB activity[12].
Previously we have demonstrated that incubation with TRAIL induces NFκB activation in hepatocellular carcinoma (HCC) cell lines, and the cells show strong resistance to TRAIL-induced apoptosis[13-17]. Therefore, we have inve-stigated the reduction of cell viability induced by inhibition of NFκB using E3330 and some chemo-therapeutic drugs or TNF family members, especially TRAIL.
E3330, a quinone derivative ((2E)-3-[5-(2,3-dimethoxy-6-methyl-1,4-benzoquinoyl)]-2-propenoic acid), was a gift from Eisai Co., Ltd. E3330 could inhibit LPS-induced TNFα generation in human monocytes[18]. TNF-family members, TRAIL, TNFα, Fas ligand were obtained from MBL, Nagoya, Japan. Chemotherapeutic agents doxorubicin and camptotecin were obtained from Sigma.
The HLE cell line was purchased from the Health Science Research Resources Bank (Osaka, Japan). HepG2 and SKHep1 cells were purchased from American Type Culture Collection (Rockville, MD, USA). Cells were cultured in Dulbecco’s modified Eagle’s medium (Dainippon Pharmaceutical Co., Ltd., Osaka, Japan) at 37 °C. All media were supplemented with 1% penicillin/streptomycin (GIBCO BRL) and 10% heat-inactivated fetal calf serum (GIBCO BRL).
The pNFκB-Luc vector (Mercury Pathway Profiling System) and the pCMV-IkBa vector were obtained from Clontech (San Diego, CA, USA). Human HCC cells (2×105) were grown in six-well plates in triplicate the day before transfection. Cells were transfected using FuGENE 6 (Boehringer Mannheim) and incubated for 18 h at 37 °C. The medium was removed and cells were incubated in complete media for 24 h. Cells were stimulated with 20 ng/mL TRAIL (R&D Systems, Nagoya, Japan), 100 U/mL TNFα (Genzyme-Techne, Cambridge, MA, USA) for 24 h. Luciferase activity was determined from cell extracts using a luciferase assay system (Promega) and a luminometer (Berthold Analytical Instruments, Nashua, NH, USA). The results were presented as the fold induction above the luciferase activity found in cells without stimulation.
To assess the viability of human HCC cell lines, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay was performed. The cells ware plated at a density of 5×103 cells/well in 96-well microtiter plates and each plate was incubated for 24 h at 37 °C in 50 mL/L CO2. HCC cell lines were treated with E3330 for 12 h and then with TRAIL (20 ng/mL), TNFα (100 U/mL), Fas ligand (500 ng/mL), doxorubicin (0.1 μg/mL) and/or camptotecin (0.1 μg/mL) for another 12 h. The live-cell count was assayed using a cell titer 96 assay kit (Promega, Madison, WI, USA) according to the manufacturer’s instructions. The absorbance of each sample was measured at 570 nm with a microtiter plate reader (Bio-Rad Laboratories, Hercules, CA, USA).
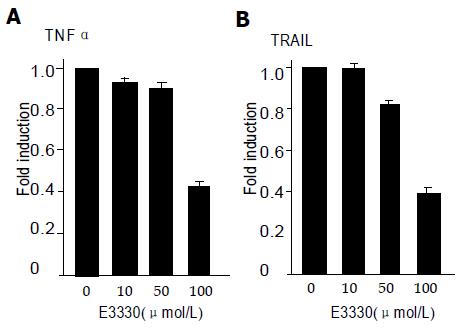
NFκB activation was induced by TNFα, TRAIL and E3330 in HLE cells. Because all human HCC cell lines showed strong resistance to TNFα- and TRAIL-mediated apoptosis, we investigated NFκB levels by NFκB luciferase reporter gene assay 12 h after NFκB inhibitor (E3330) application in HLE cell line. E3330 decreased NFκB levels in a dose-dependent manner in HLE cells stimulated by TNFα and TRAIL (Figure 1).
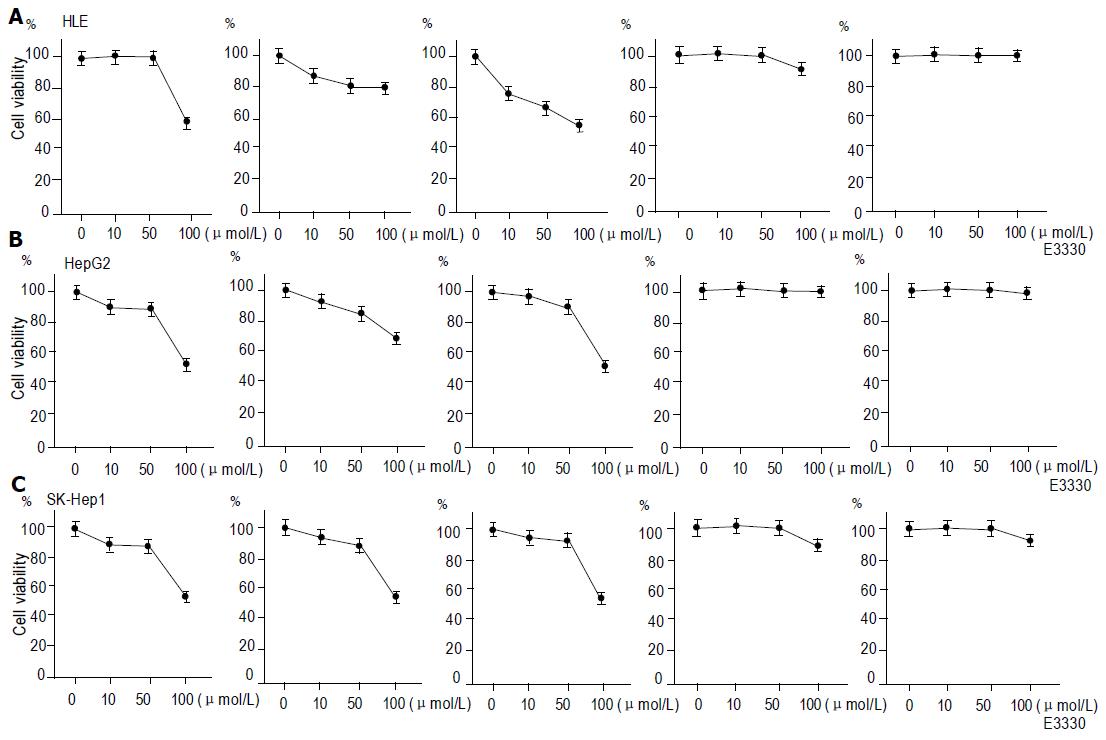
The cell viability was decreased by the combination of TRAIL, TNFα, Fas ligand, and E3330 in HCC cell lines. The effect of E3330 on apoptosis induced by TRAIL, TNFα and Fas ligand was examined because E3330 was shown to inhibit the activity of NFκB. We incubated human HCC cell lines with E3330 for 12 h, then combined E3330 with TNFα (100 U/mL), Fas ligand (500 ng/mL), or TRAIL (20 ng/mL), and examined the cell viability after 12 h by MTT assay. The cytotoxicity of the combination of TRAIL, TNFα, Fas ligand and E3330 increased synergistically in a dose-dependent manner compared to E3330 alone in all HCC cell lines (Figure 2).
Next, we investigated the cell viability of HCC cell lines inhibited by both chemotherapeutic agents and E3330. We incubated human HCC cell lines with E3330 for 12 h, then combined E3330 with doxorubicin (0.1 mg/mL) or camptotecin (0.1 mg/mL) for an additional 12 h and examined the cell viability after 12 h by MTT assay. Interestingly, doxorubicin and camptotecin had little effect on the reduction of cell viability in all HCC cell lines though they were used in combination with E3330 (Figure 2).
TRAIL can induce apoptosis by interaction with two receptors, TRAIL-R1 (DR4) and TRAIL-R2 (DR5)[19-24]. These receptors have a death domain that mediates cellular apoptosis. Two other receptors, known as TRAIL-R3 and TRAIL-R4, inhibit apoptosis by acting as decoy receptors because they do not contain the cytoplasmic death domain. These decoy receptors are highly expressed in normal tissues, but have a substantially lower expression in malignant cells[21-26].
However, it is of interest to determine at which level HCC cells inhibit TRAIL-induced death signaling in HCC cells. NFκB is a key mediator in the inhibition of apoptotic responses and NFκB activation has been shown to increase the antiapoptotic threshold of cells and tissues exposed to cytotoxic cytokines such as TNF by suppressing the initiation of caspase-8 activation[27]. On the other hand, NFκB has many wide-ranging effects that are controlled by a complex regulatory network of inhibitors and coactivators[28-30]. Given the intimate connection between host defense reactions and NFκB, this transcription factor and its associated regulators could provide attractive targets for therapeutic intervention in a number of diseases or pathologic conditions. In this line, a number of anti-NFκB drugs have already been developed[12].
In the current report, E3330 inhibits NFκB DNA binding, most probably via an interaction with a nuclear factor that activates NFκB activity[12]. We studied E3330- inhibited NFκB activity in HCC cell line and found that E3330 decreased NFκB levels in a dose-dependent manner at HLE cells stimulated by TNFα and TRAIL.
NFκB may inhibit the apoptosis induced by TNF family members such as TRAIL, TNFα, Fas ligand. Our results indicate that the inhibition of NFκB could increase cytotoxicity in combination with TNF family members such as TRAIL, TNFα and Fas ligand in HCC cell lines, while E3330 could inhibit NFκB in a dose-dependent manner. Thus, we believe that inhibition of NFκB could augment the cellular apoptosis induced by TNF family members.
Within the TNF family members, the receptor/ligand pair Fas/Fas ligand has been noted to play an important role in the apoptosis of hepatocytes. In contrast to Fas/Fas ligand, TRAIL is able to kill a broad spectrum of tumor cells, but appears to be nontoxic to most normal cells[7]. We demonstrated that inhibition of NFκB sensitized human HCC cell lines to TNF-mediated apoptosis, suggesting that TRAIL-based tumor therapy in combination with anti-NFκB agents might be a powerful potential therapeutic tool in the treatment of human HCC.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Steller H. Mechanisms and genes of cellular suicide. Science. 1995;267:1445-1449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1812] [Cited by in RCA: 1753] [Article Influence: 58.4] [Reference Citation Analysis (0)] |
| 2. | Nagata S. Apoptosis by death factor. Cell. 1997;88:355-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3739] [Cited by in RCA: 3644] [Article Influence: 130.1] [Reference Citation Analysis (0)] |
| 3. | Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4724] [Cited by in RCA: 4686] [Article Influence: 156.2] [Reference Citation Analysis (0)] |
| 4. | Schulze-Osthoff K, Ferrari D, Los M, Wesselborg S, Peter ME. Apoptosis signaling by death receptors. Eur J Biochem. 1998;254:439-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 723] [Cited by in RCA: 714] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 5. | Havell EA, Fiers W, North RJ. The antitumor function of tumor necrosis factor (TNF), I. Therapeutic action of TNF against an established murine sarcoma is indirect, immunologically dependent, and limited by severe toxicity. J Exp Med. 1998;167:1067-1085. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 204] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 6. | Ogasawara J, Watanabe-Fukunaga R, Adachi M, Matsuzawa A, Kasugai T, Kitamura Y, Itoh N, Suda T, Nagata S. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364:806-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1429] [Cited by in RCA: 1429] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 7. | Mundt B, Kühnel F, Zender L, Paul Y, Tillmann H, Trautwein C, Manns MP, Kubicka S. Involvement of TRAIL and its receptors in viral hepatitis. FASEB J. 2003;17:94-96. [PubMed] |
| 8. | Kuhnel F, Zender L, Paul Y, Tietze MK, Trautwein C, Manns M, Kubicka S. NFkappaB mediates apoptosis through transcriptional activation of Fas (CD95) in adenoviral hepatitis. J Biol Chem. 2000;275:6421-6427. [RCA] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 123] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 9. | Natoli G, Costanzo A, Guido F, Moretti F, Bernardo A, Burgio VL, Agresti C, Levrero M. Nuclear factor kB-independent cytoprotective pathways originating at tumor necrosis factor receptor-associated factor 2. J Biol Chem. 1998;273:31262-31272. [RCA] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 90] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Nagakawa J, Hirota K, Hishinuma I, Miyamoto K, Sonoda J, Yamanaka T, Katayama K, Yamatsu I. Protective effect of E3330, a novel quinone derivative, in galactosamine-induced hepatitis in rats. J Pharmacol Exp Ther. 1993;264:496-500. [PubMed] |
| 11. | Nagakawa J, Hishinuma I, Miyamoto K, Hirota K, Abe S, Yamanaka T, Katayama K, Yamatsu I. Protective effects of (2E)-3-[5-(2,3-dimethoxy-6-methyl-1,4- benzoquinoyl)]-2-nonyl-2-propenoic acid on endotoxin-mediated hepatitis in mice. J Pharmacol Exp Ther. 1992;262:145-150. [PubMed] |
| 12. | Hiramoto M, Shimizu N, Sugimoto K, Tang J, Kawakami Y, Ito M, Aizawa S, Tanaka H, Makino I, Handa H. Nuclear targeted suppression of NF-kappa B activity by the novel quinone derivative E3330. J Immunol. 1998;160:810-819. [PubMed] |
| 13. | Okano H, Shiraki K, Inoue H, Kawakita T, Yamanaka T, Deguchi M, Sugimoto K, Sakai T, Ohmori S, Fujikawa K. Cellular FLICE/caspase-8-inhibitory protein as a principal regulator of cell death and survival in human hepatocellular carcinoma. Lab Invest. 2003;83:1033-1043. [RCA] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 106] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Ito T, Shiraki K, Sugimoto K, Yamanaka T, Fujikawa K, Ito M, Takase K, Moriyama M, Kawano H, Hayashida M. Survivin promotes cell proliferation in human hepatocellular carcinoma. Hepatology. 2000;31:1080-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 264] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 15. | Suzuki A, Hayashida M, Kawano H, Sugimoto K, Nakano T, Shiraki K. Hepatocyte growth factor promotes cell survival from fas-mediated cell death in hepatocellular carcinoma cells via Akt activation and Fas-death-inducing signaling complex suppression. Hepatology. 2000;32:796-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 105] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Shiraki K, Tsuji N, Shioda T, Isselbacher KJ, Takahashi H. Expression of Fas ligand in liver metastases of human colonic adenocarcinomas. Proc Natl Acad Sci USA. 1997;94:6420-6425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 192] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 17. | Yamanaka T, Shiraki K, Sugimoto K, Ito T, Fujikawa K, Ito M, Takase K, Moriyama M, Nakano T, Suzuki A. Chemotherapeutic agents augment TRAIL-induced apoptosis in human hepatocellular carcinoma cell lines. Hepatology. 2000;32:482-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 150] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Miyamoto K, Nagakawa J, Hishinuma I, Hirota K, Yasuda M, Yamanaka T, Katayama K, Yamatsu I. Suppressive effects of E3330, a novel quinone derivative, on tumor necrosis factor-alpha generation from monocytes and macrophages. Agents Actions. 1992;37:297-304. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Pan G, O'Rourke K, Chinnaiyan AM, Gentz R, Ebner R, Ni J, Dixit VM. The receptor for the cytotoxic ligand TRAIL. Science. 1997;276:111-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1333] [Cited by in RCA: 1352] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 20. | Schneider P, Thome M, Burns K, Bodmer JL, Hofmann K, Kataoka T, Holler N, Tschopp J. TRAIL receptors 1 (DR4) and 2 (DR5) signal FADD-dependent apoptosis and activate NF-kappaB. Immunity. 1997;7:831-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 547] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 21. | Schneider P, Bodmer JL, Thome M, Hofmann K, Holler N, Tschopp J. Characterization of two receptors for TRAIL. FEBS Lett. 1997;416:329-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 224] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 22. | MacFarlane M, Ahmad M, Srinivasula SM, Fernandes-Alnemri T, Cohen GM, Alnemri ES. Identification and molecular cloning of two novel receptors for the cytotoxic ligand TRAIL. J Biol Chem. 1997;272:25417-25420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 425] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 23. | Pan G, Ni J, Wei YF, Yu G, Gentz R, Dixit VM. An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science. 1997;277:815-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1163] [Cited by in RCA: 1165] [Article Influence: 41.6] [Reference Citation Analysis (1)] |
| 24. | Sheridan JP, Marsters SA, Pitti RM, Gurney A, Skubatch M, Baldwin D, Ramakrishnan L, Gray CL, Baker K, Wood WI. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science. 1997;277:818-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1275] [Cited by in RCA: 1291] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 25. | Pan G, Ni J, Yu G, Wei YF, Dixit VM. TRUNDD, a new member of the TRAIL receptor family that antagonizes TRAIL signalling. FEBS Lett. 1998;424:41-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 233] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 26. | Degli-Esposti MA, Dougall WC, Smolak PJ, Waugh JY, Smith CA, Goodwin RG. The novel receptor TRAIL-R4 induces NF-kappaB and protects against TRAIL-mediated apoptosis, yet retains an incomplete death domain. Immunity. 1997;7:813-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 643] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 27. | Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS. NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680-1683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2159] [Cited by in RCA: 2188] [Article Influence: 81.0] [Reference Citation Analysis (0)] |
| 28. | Grilli M, Chiu JJ, Lenardo MJ. NF-kappa B and Rel: participants in a multiform transcriptional regulatory system. Int Rev Cytol. 1993;143:1-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 676] [Cited by in RCA: 741] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 29. | Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3598] [Cited by in RCA: 3640] [Article Influence: 117.4] [Reference Citation Analysis (1)] |
| 30. | Siebenlist U, Franzoso G, Brown K. Structure, regulation and function of NF-kappa B. Annu Rev Cell Biol. 1994;10:405-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1634] [Cited by in RCA: 1654] [Article Influence: 53.4] [Reference Citation Analysis (0)] |