Published online Oct 21, 2005. doi: 10.3748/wjg.v11.i39.6110
Revised: December 23, 2004
Accepted: December 29, 2004
Published online: October 21, 2005
AIM: To investigate the relation between the expression of cyclooxygenase-2 (COX-2) and liver cancer, to construct the recombinant adenovirus encoding human COX-2 antisense RNA, and to explore its effects on liver cancer cell proliferation.
METHODS: We studied the expression of COX-2 in 34 cases of hepatocellular carcinoma (HCC) and SMMC7402 and SMMC7721 by immunohistochemical technique. Recombinant adenovirus Ad-AShcox-2 was constructed and transfected into human HCC cell lines SMMC7402 and SMMC7721, and its effects on COX-2 expression, cell apoptosis and cell cycle were analyzed by flow cytometry. Cell proliferation was determined by colony-forming efficiency.
RESULTS: We observed COX-2 expression in 82.4% of HCC and SMMC7402 cells, but no COX-2 expression in SMMC7721 cells. In addition, recombinant adenovirus encoding antisense COX-2 fragment Ad-AShcox-2 was obtained with the titer of 1.06×1012 PFU/mL. Ad-AShcox-2 could reduce the expression of COX-2 and enhance the percentage of cells in G1/G0 phase in SMMC7402 cell line. The difference of apoptotic index between the Ad-AShcox-2 group and control group was statistically significant (tcontrol group = 32.62 and tAd-LacZ = 10.93, P<0.001) in SMMC7402 but not in SMMC7721. Similarly, colony-forming rates of SMMC7402 and SMMC7721 cell lines, after the transfer of Ad-AShcox-2, were (2.7±0.94)% and (33.6±4.24)%, respectively.
CONCLUSION: Reduction in the expression of COX-2 can inhibit COX-2 expressing HCC cells.
- Citation: Wang XH, Li SB, Tong Q, Xie GJ, Wu QM. Effects of adenovirus-mediated human cyclooxygenase-2 antisense RNA on the growth of hepatocellular carcinoma. World J Gastroenterol 2005; 11(39): 6110-6114
- URL: https://www.wjgnet.com/1007-9327/full/v11/i39/6110.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i39.6110
Liver cancer is a kind of malignant tumor with a high mortality at present. Its morbidity is rather high in our country. Although a great number of researches on liver cancer as well as many achievements have been made, the exact molecular pathogenesis of liver cancer is not clear. Cyclooxygenase-2 (COX-2) has a high expression in quite a few tumors such as colorectal cancer[1,2] esophageal cancer[3], and other cancers[4]. It is likely to be related to the genesis and development of tumor, but the causing-carcinoma effect can be resisted by COX-2 selective inhibitors[5-7]. Our prophase researches showed that COX-2 antisense RNA obviously inhibits the growth of carcinoma of esophagus with a high expression of COX-2[8,9]. COX-2 is likely to become a new target of treating tumor. The present study aimed to investigate the relationship between the expression of COX-2 and liver cancer, to the construction of the recombinant adenovirus encoding human COX-2 anti-sense RNA, and to treat hepatocellular carcinoma (HCC) with or without the expression of COX-2 and to explore its mechanism.
BamHI, XbaI, and T4 DNA ligase were purchased from Huamei Biological Engineering Company. RPMI 1640 and Lipofectamine were provided by GIBCO/BRL. CsCl was from Sigma Company. COX-2 cDNA and primer of adenovirus PCR were designed and synthesized by Saibaisheng Biology Company.
Ad-LacZ was a gift from Dr. Jia-Ning Wang in our Molecular Biological Laboratory. COX-2 plasmid PCRTMII vector was supplied by Dr. You-Fei Guan in Vanderbilt University, USA. pHCMVSP1A and pJM17 were donated by academician Wu Zuze in the Second Institute of Academy of Military Medical Sciences. DH5a was donated by Dr. Peng Xu in Cardiology Internal Medicine Department of No. 1 Hospital Peking University. 293 cells and HCC cell lines SMMC7721 and SMMC7402 were purchased from Shanghai Cell Bank of Chinese Academy of Medical Sciences.
High-speed refrigerated centrifuge (Jouan, Italy), carbon dioxide incubator (Sany D, Japan), ultrahigh refrigerated centrifuge (Hitachi, Japan), inversion difference microscope (Olympus IX-To, Japan), PCR amplifier (Amplitron II, USA), flow cytometer (EPICS XL, Beckman Coulter Company, USA), ultraviolet spectrophotometer (752, Shanghai), air bath, and water bath cradle were used in our study.
Immunohistochemical transfection was conducted by streptomy-glair-in-biotin-horseradish peroxidase (SP) compound method. The tissue was fixed with paraffin and cut into 5-mm-thick sections. HCC tissue slides were fixed in 4% PFA in PBS for 10 min, washed twice with PBS, and incubated in 3% H2O2 to eliminate the endogenous peroxidase activities. The tissues were then incubated either at room temperature for 1 h or at 4 °C overnight with 1% goat serum in PBS to block the nonspecific binding of antibodies. The slides were further incubated sequentially with rabbit anti-man COX-2 polyclonal antibody. PBS was used as a control. Color development was performed by incubation with 3,3-diaminobenzidine tetrahydrochloride in 0.03% H2O2 and 50 mmol/L Tris-HCl, pH 7.4. The percentage of positive tumor cells was determined semiquantitatively by assessing the whole tumor section, and each sample was assigned to one of the following categories: 0 (0-4%), 1 (5-24%), 2 (25-49%), 3 (50-74%), 4 (75-100%). Transfection strength was indicated with 0 (negative), 1 (weak positive), and 2 (positive). The expression levels were indicated with positive rate of tumor cells of one tumor tissue showing the different transfection strength. Its expression level was the transfection strength fraction by positive rate fraction. For example, if 50 tumor cells of one tumor tissue showed positive staining, we could mark 3 by 2 equaling 6; if 25 showed weak positive staining, we could mark 2 by 1 equaling 2; if 25% showed negative staining, we could mark 2 by 0 equaling 0. Thus the total was 6 plus 2 plus 0 equaling 8. The specific result statistics were carried out as previously described[10].
First, we obtained a COX-2 cDNA fragment using XbaI and BamHI to bi-mold-cut the plasmid PCRTMIICOX-2, and cloned the COX-2 cDNA fragment in the reverse direction onto two spots of XbaI and BamHI of the pHCMUSP1A, the connection product was pAd-Ashcox-2. Then the latter was plasmid-amplified by transformed bacillus coli DH5a and identified as positive plasmid by mold-cutting. The shuttle plasmid pAd-Ashcox-2 mediated Lipofectamine and pJM17 were co-transferred into 293 cells. When 293 cells had pathologic lesions and exfoliative cells made up 20%, we collected the upper clear liquid. Then we extracted viral DNA from the upper clear liquid and identified it by PCR. COX-2 primer's upstream sequences are 5'-TTG GCT TCA AGA CTG AGA TA-3'and 5'-AGC CCA AAT TAT TGG TTC-3'.Adenovirus primer's upstream sequence is 5'-TCG TTT CTC AGC AGC TGT TG-3', downstream sequence is 5'-CAT CTG AAC TCA AAG CGT GG-3'. PCR reaction system contained 10 μL viral DNA, 10 μL 20 μmol/L primer respectively (5 μL adenovirus), 5 μL 10×PCR buffer, 2 μL MgCl2, 5 μL 2 mmol/L dNTP and 1 μL Taq enzyme. Water was added until the final volume of PCR reaction was 50μL. Thirty cycles of PCR were carried out, each consisting of 30 s at 94 °C, 30 s at 56 °C, 1 min at 72 °C, then a final extension at 72 °C for 10 min. Electrophoresis in 1.0% agarose gel was performed to amplify cDNA and adenovirus fragments as positive reconstructive cloning. Without adding mould, PCR amplification was used as a negative control. After positive reconstructive adenovirus was identified, we transferred 293 cells again. 293 cells were collected, refrigerated, melted, fragmented and ultracentrifuged twice with CsCl, viruses were collected and purified. At last the virus titer was assayed.
The recombinant adenovirus Ad-LacZ with genes encoding LacZ was used to infect liver cancer cell lines SMMC7721 and SMMC7402 according to multiplicity of infection-MOI 25, 50, 100, and 200. After 48 h, the cells were fixed and stained with X-gal. The number of cells stained blue was recorded under microscope. Then the percentage of cells with blue staining was calculated.
Human liver cancer cell lines SMMC7721 and SMMC7402 were incubated in a 50 mL/L CO2 at 37 °C. When the density reached 40-50%, the medium was replaced by serum-free RPMI 1640 and incubated for 24 h. The experimental group infected the two cell lines with 100MOI, and was incubated for 2 h with serum. During the process, the culture fluid was shaken every 15 min. Two hours later, the medium was replaced by 10% FCS RPMI 1640. The control groups were Ad-LacZ group and non-virus group. Carcinoma cells were incubated for 24, 48, 72, 96 h respectively, fixed for 10 min in 4 °C cold acetone, and dried for 10 min at room temperature. Then the cells were stored at -85 °C. The experiment was carried out with SP immunohistochemical method.
When the density of the two cell lines reached 40-50%, the medium was replaced by RPMI 1640 containing serum and incubated for 24 h. Forty-eight hours after the experimental group and control group finished virus transfection, the cells were collected (using trypsin digestion method) and centrifuged (1000 r/min, 5 min). The upper clear fluid was discarded, PBS was added to adjust cell density to 106/mL. One hundred microliters of cell suspension was put into the tube, 200 μL DNA-PREPTMLPR was added to it and mixed. Two microliters DNA-PREPTM stain reagent (PI stain) was added and mixed after 30 s. After 30 min, the cell cycle and apoptosis was detected with flow cytometry (FCM).
The two cell lines was digested into single-cell suspension respectively, and inoculated into a six-well board. Each well was inoculated with 500 cells. Each group had three pang-punches. After being treated with culture fluid Ad-AShcox-2, they were incubated for 10 d and fixed with 1:3 acetic acid:methanol, stained with Rui stain fluid. Then the number of colonies with more than 50 cells was recorded.
Expression level of COX-2 in liver cancer tissue was expressed as mean±SD. t-Test was used to show the cell apoptosis rate and cell cycle distribution rate.
COX-2 was positive in 28 of 34 cases of liver cancers and was negative in 6 of 34 cases of liver cancer. Positive rate was 82.4%. The positive staining of COX-2 was mainly in cytoplasm. High expression of COX-2 was correlated with clinical pathological features of liver cancer (Figure 1). COX-2 expression level was high, if the cancer cells were well differentiated. COX-2 expression was not related to AFP levels, source cells and transference in liver (Table 1).
| Clinical pathologicalfeatures | n | Stain fraction | t | P |
| AFP (ng/mL) | ||||
| <20 | 4 | 6.48+1.46 | 0.74 | >0.05 |
| ≥20 | 24 | 6.36+1.15 | ||
| Cell category | ||||
| Liver cell category | 27 | 6.79+1.60 | ||
| Bile duct cell category | 1 | 6.26+1.25 | ||
| Transference in liver | ||||
| Yes | 3 | 6.43+1.42 | 1.08 | <0.05 |
| No | 25 | 7.15+1.80 | ||
| Pathological differentiation | ||||
| High | 22 | 8.25+0.86 | 5.23 | <0.05 |
| Middle and low | 6 | 6.11+102 | ||
DNA was extracted and precipitated, then PCR amplification was performed on this DNA model. The product of PCR amplification was evaluated with 0.8% agarose electrophoresis, and 277-bp COX-2 cDNA fragments and 860-bp virus gene frame fragments were obtained. After pAd-AShcox-2 and pJM17 were co-transferred to 293 cells, we produced anti-sense RNA recombinant adenovirus which could express COX-2.
After Ad-AShcox-2 was amplified, extracted, purified, and assayed with ultraviolet spectrophotometer, the virus titer was 1.06×1012 PFU/mL. When the MOI was 50 or greater, the transfection rate of SMMC7721 and SMMC7402 reached 100%.
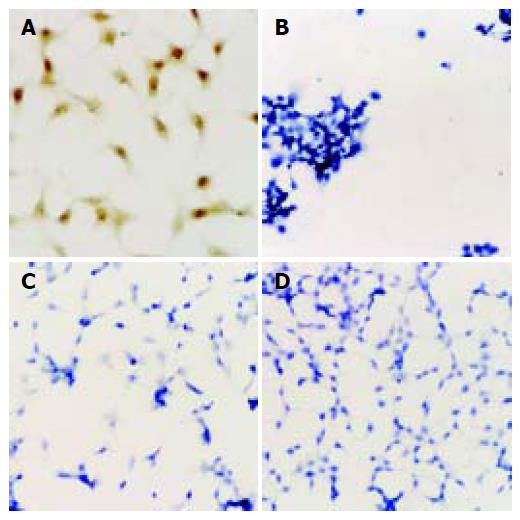
Immunohistochemistry showed that SMMC7402 had a high expression of COX-2. But after Ad-AShcox-2 was transferred, the expression of COX-2 decreased obviously. Before and after Ad-AShcox-2 was transferred, SMMC7721 did not express COX-2 (Figures 1A-D).
Forty-eight hours after SMMC7402 cells were treated with Ad-AShcox-2, FCM showed that apoptotic cells reached apoptosis peak prior to G0/G1 peak. The apoptosis rates of Ad-AShcox-2, Ad-LacZ, and the control group were (33.3±3.02)%, (0.42±0.14)%, and (0.25±0.08)%, respectively. The apoptosis rate of the experimental group increased obviously in comparison to the control group. However, after SMMC7721 was treated with Ad-AShcox-2, we could not detect the apoptosis peak with FCM. The apoptosis rates were (0.61±0.25)%, (0.48±0.09)%, and (0.56±0.11)%, respectively. The apoptosis rates of these two liver cancer cell lines, induced by Ad-AShcox-2, differed sharply when compared to each other (t = 32.05, P<0.001).
Cell cycle from FCM is listed in Table 2. After SMMC7402 cells were treated with Ad-LacZ and non-virus culture fluid, the cell proliferation rate decreased in G0/G1 phase, but it was high in S phase indicating that the proliferation of cells was active. After they were treated with Ad-AShcox-2, the number of cells in G0/G1 phase increased obviously, while in S phase, the number of cells decreased, suggesting that there was a significant difference compared to control group (P<0.05). The cells were blocked in G1 phase. But after SMMC7721 was treated with Ad-AShcox-2, the cell proliferation rates in G1/G0 phase were low both in experimental group and in control group (Table 3); yet they were high in S phase. There was no difference (P>0.05) when compared to each other.
| Groups | Apoptosis rate | Cell cycle (%) | ||
| (%) | G0/G1 | S | G2/M | |
| Control group | 0.25 | 40.39+3.86 | 49.61+4.27 | 13.10+1.03 |
| Ad-LacZ | 0.42 | 40.17+4.03 | 50.18+3.66 | 12.57+2.85 |
| Ad-ASHcox-2 | 33.3 | 66.52+5.48 | 22.15+3.26 | 10.33+1.12 |
| Groups | Apoptosis rate | Cell cycle (%) | ||
| (%) | G0/G1 | S | G2/M | |
| Control group | 0.56 | 40.39+3.86 | 49.61+4.27 | 13.10+1.03 |
| Ad-LacZ | 0.48 | 42.17+2.63 | 50.26+2.85 | 12.27+2.72 |
| Ad-ASHcox-2 | 0.91 | 39.92+4.11 | 49.28+4.13 | 12.64+1.25 |
After Ad-AShcox-2 infected SMMC7721 and SMMC7402, the colony-forming rates were (2.7±0.94)% and (33.6±4.24)%. There were significant differences between the two groups (P <0.05, n = 6).
Cyclooxygenase is a kind of a rate-limiting enzyme, which catalyzes and synthesizes prostaglandin. It has two isomers -COX-1 and COX-2. COX-1 is constitutively expressed in most mammalian tissues and is thought to carry out "housekeeping"functions such as cytoprotection of the gastric mucosa, regulation of renal blood flow, and control of platelet aggregation. In contrast, COX-2 mRNA and protein are normally undetectable in most tissues, but can be rapidly induced by proinflammatory or mitogenic stimuli including cytokines, endotoxins, interleukins, and phorbolester. It has high expression levels in many cancer tissues. Researches showed that COX-2 can promote the formation and opening of tumor vessels[11,12], inhibit tumor immunity[13,14], stimulate transference of tumors and inhibit apoptosis[15-18].
Studies indicate that COX-2 selective inhibitor can inhibit proliferation of colon cancer cells[19,7], and induce their apoptosis[20,21]. In our prophase researches, by using the carrier of COX-2 antisense RNA adenovirus-transferred esophageal carcinoma cells, we successfully inhibited the expression of COX-2 in esophageal carcinoma cells[8,9]. The growth and proliferation of esophageal carcinoma cells were obviously inhibited, showing that reducing the expression of COX-2 or inhibiting the activity of COX-2 is likely to become a new way to treat tumors. COX-2 is strongly positive in 28 of 34 cases of HCC. The positive rate reached 82.4%, suggesting that the high expression of COX-2 is related to the carcinogenesis and development of HCC. We also found that most HCCs with positive expression of COX-2 were well differentiated, but poorly differentiated HCC had a low expression of COX-2, indicating that COX-2 can promote the proliferation and differentiation of liver cancer cells, which is in line with the report of Koga et al[22]. We can infer that HCC is related to COX-2 inhibiting cell apoptosis and increasing cytoplasmic matrix protein.
We successfully constructed the recombinant adenovirus expressing COX-2 anti-sense RNA. When the two liver cancer cells were treated with it, the expression of COX-2 decreased obviously. Meanwhile, the growth of cells was inhibited and the colony-forming rates of cells decreased apparently as well. But the colony-forming rates of SMMC7721 did not decrease, indicating that inhibiting the expression of COX-2 could inhibit the growth of liver cancer cells with positive expression of COX-2. However, cells with negative expression of COX-2 could not be inhibited. We further confirmed that the feasibility of COX-2 independent way in which NSAIDs could inhibit the colon cancer cells without the expression of COX-2[23].
After SMMC7402 was transferred in Ad-AShcox-2, the rate of cell apoptosis increased notably. There was a significant difference when control group, Ad-LacZ treatment group, and blank group were compared to each other (P<0.001), but the apoptosis rate of SMMC7721 did not increase, showing that by reducing the expression of COX-2, we can increase the apoptosis of cancer cells. This is in line with the result of a previous study[17].
Cell cycle test showed that Ad-AShcox-2 could reduce the expression of COX-2 in SMMC7402, the cell number increased evidently in G0/G1 phase. It could prevent cells passing from G1 to S phase. It is likely to be linked with the expression of raised P27 and the inhibition of cyclin/CDK activity[24,25].
In conclusion, the adenovirus carrier with the expression of COX-2 anti-sense RNA is able to inhibit SMMC7402 and induce apoptosis.
Science Editor Wang XL and Zhu LH Language Editor Elsevier HK
| 1. | Giardiello FM, Spannhake EW, DuBois RN, Hylind LM, Robinson CR, Hubbard WC, Hamilton SR, Yang VW. Prostaglandin levels in human colorectal mucosa: effects of sulindac in patients with familial adenomatous polyposis. Dig Dis Sci. 1998;43:311-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 70] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Zhan J, Liu JP, Zhu ZH, Yao HR, Chen CY. Relationship between COX-2 expression and clinicopathological features of colorectal cancers. Chin Med J (Engl). 2004;117:1151-1154. [PubMed] |
| 3. | Wu QM, Li SB, Wang Q, Wang DH, Li XB, Liu CZ. The expression of cox-2 in esophageal carcinoma and its realation to clinicopathologic characteristic. Shijie Huaren Xiahua Zazhi. 2001;1:11-14. |
| 4. | Takahashi T, Kozaki K, Yatabe Y, Achiwa H, Hida T. Increased expression of COX-2 in the development of human lung cancers. J Environ Pathol Toxicol Oncol. 2002;21:177-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Dang CT, Shapiro CL, Hudis CA. Potential role of selective COX-2 inhibitors in cancer management. Oncology (Williston Park). 2002;16:30-36. [PubMed] |
| 6. | Howe LR, Dannenberg AJ. A role for cyclooxygenase-2 inhibitors in the prevention and treatment of cancer. Semin Oncol. 2002;29:111-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Minter HA, Eveson JW, Huntley S, Elder DJ, Hague A. The cyclooxygenase 2-selective inhibitor NS398 inhibits proliferation of oral carcinoma cell lines by mechanisms dependent and independent of reduced prostaglandin E2 synthesis. Clin Cancer Res. 2003;9:1885-1897. [PubMed] |
| 8. | Wu QM, Li SB, Liu CZ, Wang XH, Xie GJ, Yu JP. The effects of adenovirus-mediated human COX-2 antisense RNA on growth of esophageal carcinoma cells. Zhongguo Zhongliu Shengwu Zhiliao Zazhi. 2001;8:285-289. |
| 9. | Li SB, Wu QM, Wang Q, Wang XH, Xie GJ. Effects of adenovirus-mediated human cox-2 antisense RNA on synthesis of DNA and proteins in esophageal carcinoma cell line. Shijie Huaren Xiahua Zazhi. 2003;5:517-521. |
| 10. | Krajewska M, Krajewski S, Epstein JI, Shabaik A, Sauvageot J, Song K, Kitada S, Reed JC. Immunohistochemical analysis of bcl-2, bax, bcl-X, and mcl-1 expression in prostate cancers. Am J Pathol. 1996;148:1567-1576. [PubMed] |
| 11. | Leung WK, To KF, Go MY, Chan KK, Chan FK, Ng EK, Chung SC, Sung JJ. Cyclooxygenase-2 upregulates vascular endothelial growth factor expression and angiogenesis in human gastric carcinoma. Int J Oncol. 2003;23:1317-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1650] [Cited by in RCA: 1644] [Article Influence: 60.9] [Reference Citation Analysis (0)] |
| 13. | Kusumoto H, Maehara Y, Anai H, Kusumoto T, Sugimachi K. Potentiation of adriamycin cytotoxicity by dipyridamole against HeLa cells in vitro and sarcoma 180 cells in vivo. Cancer Res. 1988;48:1208-1212. [PubMed] |
| 14. | Kakiuchi Y, Tsuji S, Tsujii M, Murata H, Kawai N, Yasumaru M, Kimura A, Komori M, Irie T, Miyoshi E. Cyclooxygenase-2 activity altered the cell-surface carbohydrate antigens on colon cancer cells and enhanced liver metastasis. Cancer Res. 2002;62:1567-1572. [PubMed] |
| 15. | Hamasaki Y, Kitzler J, Hardman R, Nettesheim P, Eling TE. Phorbol ester and epidermal growth factor enhance the expression of two inducible prostaglandin H synthase genes in rat tracheal epithelial cells. Arch Biochem Biophys. 1993;304:226-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Jones DA, Carlton DP, McIntyre TM, Zimmerman GA, Prescott SM. Molecular cloning of human prostaglandin endoperoxide synthase type II and demonstration of expression in response to cytokines. J Biol Chem. 1993;268:9049-9054. [PubMed] |
| 17. | Tsujii M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell. 1995;83:493-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1569] [Cited by in RCA: 1565] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 18. | Aoki T, Tsukinoki K, Karakida K, Ota Y, Otsuru M, Kaneko A. Expression of cyclooxygenase-2, Bcl-2 and Ki-67 in pleomorphic adenoma with special reference to tumor proliferation and apoptosis. Oral Oncol. 2004;40:954-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Kawamori T, Rao CV, Seibert K, Reddy BS. Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, against colon carcinogenesis. Cancer Res. 1998;58:409-412. [PubMed] |
| 20. | Grösch S, Tegeder I, Niederberger E, Bräutigam L, Geisslinger G. COX-2 independent induction of cell cycle arrest and apoptosis in colon cancer cells by the selective COX-2 inhibitor celecoxib. FASEB J. 2001;15:2742-2744. [PubMed] |
| 21. | Richter M, Weiss M, Weinberger I, Fürstenberger G, Marian B. Growth inhibition and induction of apoptosis in colorectal tumor cells by cyclooxygenase inhibitors. Carcinogenesis. 2001;22:17-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 96] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Koga H, Sakisaka S, Ohishi M, Kawaguchi T, Taniguchi E, Sasatomi K, Harada M, Kusaba T, Tanaka M, Kimura R. Expression of cyclooxygenase-2 in human hepatocellular carcinoma: relevance to tumor dedifferentiation. Hepatology. 1999;29:688-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 303] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 23. | Tsujii M, Kawano S, DuBois RN. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci USA. 1997;94:3336-3340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 997] [Cited by in RCA: 1039] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 24. | DuBois RN, Shao J, Tsujii M, Sheng H, Beauchamp RD. G1 delay in cells overexpressing prostaglandin endoperoxide synthase-2. Cancer Res. 1996;56:733-737. [PubMed] |
| 25. | Hung WC, Chang HC, Pan MR, Lee TH, Chuang LY. Induction of p27(KIP1) as a mechanism underlying NS398-induced growth inhibition in human lung cancer cells. Mol Pharmacol. 2000;58:1398-1403. [PubMed] |