INTRODUCTION
Portal hypertension is characterized not only by lesions in liver, but also by the changes in vascular structures and functions of extrahepatic portal system, systemic system and pulmonary circulation. During a long-term animal experiment as well as in clinical practice, Yang et al[1] discovered that obvious pathological changes occurred in visceral vessels with the increment of the portal pressure, such as formation of extensive portal-systemic communicating branches, the changes in modeling of visceral veins and arteries, etc., which were named vasculopathy in portal hypertension. Generally, vascular lesions also include microvascular lesions in viscera, especially in stomach and intestine, named as portal hypertensive gastropathy and portal hypertensive intestinal vasculopathy, respectively, which can cause hematochezia, occult blood in stool or hemorrhage of digestive tract. Portal hypertension can also be complicated with pulmonary vascular lesions, leading to pulmonary hypertension and elevation of pulmonary venous pressure, the former might contribute to the inevitable injury of pulmonary arterioles and arteries due to the passage of vasoactive substances into lung through collateral circulation, as well as the synthesis of vasoactive substances produced by lung itself, while the latter can result in varicose vein in lung, trachea and bronchi, and hemorrhage following the disruption of the varicose veins[2,3]. Perforating vein is the special vascular structure of gastric wall in portal hypertension[4], and is considered as a vascular lesion. The connection of varicose vessels outside gastric tunica serosa with the perforating vein leads to the abnormal flow into the submucous layer of the gastric wall to form the varicose vein. In this paper, we have briefly reviewed the research progress in the pathology and pathogenesis of vasculopathy in portal hypertension.
Pathology of vascular lesions in portal hypertension-induced remodeling of arterialized visceral veins
The changes in visceral venous structures, such as collagen, elastic fibers, and smooth muscles, are involved in the vein lesions. Under light microscope, the smooth muscle cells in portal wall display hypertrophy and hyperplasia. Under electron microscope, the traverse areas of the smooth muscle cells have onefold increase as compared with the normal one and that of vascular media is increased subsequently. The rat model of partial portal ligation revealed the changes in vascular structure, contractility, contractile components and structural proteins, and the proportion of desmin and actin was found to be increased. The thickened vascular intima contained hyperplastic collagenous fibers and large numbers of disordered smooth muscles. The interstitial was also obviously thickened with thickened and condensed muscle fibers. In the adventitial layers, there was infiltration of inflammatory cells and onefold increase in the active contractility[5].
The arterialized remodeling exists in both splenic veins and coronary vein of stomach in the patients with portal hypertension[6], which includes: (1) Obviously thickened intima, showing diffuse thickening and locally thickened areas, forming neointima containing hyperplastic collagenous fibers and large numbers of disordered smooth muscle cells. The hillock-shaped hyperplastic spots extrude towards the vascular cavity, also containing large numbers of collagenous fibers and disordered smooth muscle cells. Platelet aggregation and blood cell attachment to the surface of injured endothelial cells gradually lead to the formation of microthrombi, similar to the plaque of atherosclerosis. The vascular intima is widely injured with infiltration of inflammatory cells; (2) The interstitial layer is significantly thickened, internal circular muscles are arranged lengthwise and breadthwise, and longitudinal muscles undergo up-growth with thickened and condensed muscle fibers; (3) There is infiltration of inflam-matory cells in the adventitial layer and few lymphatic nodes are complicated with inflammation. The feeding vessels are squeezed with the formation of thrombi; (4) The phenotypes of part muscle cells convert from contractile type to synthetic type, that is, many rough reticulum and ribosomes and decreased myofilaments exist in the cytoplasm, and migrate from interstitia to intima.
Intimal injury of visceral veins
Vascular endothelial cell, attaching to the surface of vascular intima, is a semi-transparent barrier membrane and possesses complicated metabolic and endocrine functions simultaneously. Researchers are paying attention to the importance of endothelial cells in cardiovascular diseases increasingly, but the correlation between endothelial cells and portal hypertension has not been elucidated clearly. It has been confirmed that the vascular intima in the patients with non-hepatic cirrhosis is intact, there is a thin layer of smooth muscle in subintima, and the feeding vessels are intact and open. While in the patients with hepatic cirrhosis, the vascular intima is severely injured, endothelial cells are detached, the subintima is nude and edematous, and there are large numbers of inflammatory cells and fibroblasts in the vascular cavity, many blood cells adhere to the nude subintima, and mural thrombi form in the vascular cavity. In the vascular adventitia, the feeding vessels become flat by extrusion and thrombi formation also takes place. McCarthy et al[7] found that when the vascular endothelial cells and basement membrane were injured, the sensitivity of vessels to angiotensin-II (AngII) was increased, suggesting that the damage to the intima could influence the contractile function of the vessels.
Destruction of contractile structure in visceral arterial wall
The patients with portal hypertension are in a state of high flow and low resistance in systemic circulation (i.e., the cardiac output and arterial pressure are decreased). Moreover, they are commonly associated with splenomegaly and hypersplenism. The splenic artery has obvious histopathological changes, i.e., destruction of contractile structure in arterial wall[8] as follows: (1) Damage of arterial endothelial cells. Partial endothelial cells detach, platelets attach to intima, which is thickened and the smooth muscles in interstitia migrate to it; (2) Weigert staining certifies that the folded ripples from elastic membrane in arterial wall become flat or completely disappear. The elastic membrane is split into multiple layers. Some parts are ruptured completely. The elastic fibers in arterial wall are also diffusely ruptured and degenerated. Under an electron microscope, split, even partly ruptured elastic membrane, is clearly seen; (3) Under light microscope, the smooth muscles in interstitia can be seen to be migrated to intima. Some medial smooth muscle cells are atrophic and anatrophic, others are hypertrophic and hyperplastic. The smooth muscle cells are separated by collagenous fibers, and arranged disorderly. Under electron microscope, the size and morphology of the smooth muscle cells are uneven, i.e., some are atrophic and anatrophic, vacuoles form in the necrotized local cytoplasm, and even whole muscle cell is lysed, while others are compensatively hypertrophic and hyperplastic. In some muscle cells, cytoplasmic cellular organs are obviously increased, mitochondria are increased and swollen, and their cristae disappear with the formation of vacuoles. In others, there exist many rough reticulum, ribosomes and Golgi bodies in cytoplasm, while the filaments are decreased, indicating that the phenotypes of muscle cells convert from contractile type to synthetic type. The cytoplasm and nuclei in some muscle cells display the characteristics of cell apoptosis; (4) Under light microscope, collagenous fibers and extracellular matrix in whole vascular wall are increased, leading to collagenization of vascular wall, and its thickening and rigidness, and decreased elasticity. Under electron microscope, the extracellular matrix in arterial wall is obviously increased, but the elastic fibers are degenerated and ruptured.
Mechanism of vasculopathy in portal hypertension
The mechanism of vasculopathy in portal hypertension is not clear. The changes in hemodynamics of portal system, immune responses, gene regulation, and vasoactive substances might contribute to the process of vasculopathy in portal hypertension.
Changes in portal hemodynamics
Vascular wall, composed of different biomaterials, possesses multiple layers of compound structure. Within a certain pressure range, vascular tension, transmural blood pressure, and the geometrical parameters follow the Laplace's law of biomechanics:T = P(R/W). The vascular tension (T) has its regulatory limitation. Within an elastic range, when the transmural blood pressure (P) is increased, compensation can be achieved by increasing the vascular width (W) and decreasing vascular radius (R). So, the high pressure of portal vein can thicken the vascular wall.
The increase of vascular resistance in portal system at any part can result in portal hypertension. Neurohumoral factors and vasoactive substances can actively regulate the contractility of vascular smooth muscles. The active changes in vascular resistance can happen in hepatic microcirculation, also in prehepatic portal-systemic communicating vessels, portal vein and their branches. Effect on vascular resistance can be expressed by Poiseuille law: r = 8 mL/ r4, in which m represents the viscosity coefficient of blood, L the length of vessel and r the radius. It can be seen that the changes in vascular radius of extrahepatic portal system are the main factors influencing the resistance of portal resistance. Intimal hyperplasia, spot formation, internal wall roughness, decreased elasticity and contractility in visceral veins can limit the vascular diastolization, promote the mural thrombosis, occlude vessels and narrow vascular cavity, leading to hypostasis, even obstruction, and finally increasing the blood flow resistance. When visceral veins and portal-systemic communicating vessels are contracted, the portal resistance is obviously increased. The collateral vessels are flexural and tortuous, so their increases in length can also significantly increase the blood flow resistance. The changes of hemoglobin and plasma protein concentration in portal blood, especially the increased blood viscosity following gastroin-testinal hemorrhage, can also increase the blood flow resistance in portal veins.
Hypertrophy and hyperplasia of vascular smooth muscle cells are the important features of vasculopathy in portal hypertension. Krieger and Dzau[9] found that the increase in mechanical load of vessels could directly induce the expression of myosin gene, increase the expression of RNA and protein, and finally induce the growth of muscle cells. It is suggested that the hyperplasia and hypertrophy of vascular smooth muscle cells may be one of the adaptive compensatory responses of vessels to increased pressure.
Persistent stimulation of high pressure and blood flow of portal veins may be the main factors responsible for the vascular intimal injury of the patients with portal hypertension. Compression of feeding vessels can decrease the supply of blood to vascular wall and partial pressure of oxygen, causing ischemic damage and nutrient disturbance of vascular intima. In hepatic cirrhosis, dysfunction of liver and kidney, decreased clearance of oxygen-free radical, and increased injurants and oxygen-free radicals in portal blood can directly injure the vascular intima.
Immune response
Researchers pay increasingly more attention to the roles that vascular endothelial cells play in the pathogenesis of cardiovascular diseases. The damages to endothelial cells in portal hypertension can destroy the structure of vascular wall, cause dysfunction, influence substance exchange and result in inflammation and immune response. In the patients with hepatic cirrhosis, the cells are markedly increased in the peripheral tissue around vessels, mainly lymphocytes and inflammatory cells, associated with the proliferation of lymph follicles. Immunohistochemistry reveals that there exists strong expressions of platelet-derived growth factor, basic fibroblastic growth factor, epidermal growth factor, transforming growth factor-α (TGF-α), etc., on the vascular wall of gastric coronary vein in the patients with portal hypertension[10], indicating that the elevated pressure in gastric coronary vein can activate the smooth muscle cells of vascular wall to release many kinds of vasoactive substances. These growth factors can stimulate the proliferation, differentiation, and migration of vascular smooth muscle cells in turn, resulting in abnormal metabolism of collagenous fibers and elastic fibers. The phenotypic shift of smooth muscle cells is a chronic response to mechanical stimulation, which can induce not only cell migration, but also the synthesis of collagen and elastin, leading to the thickening of venous wall, decreased elasticity and compliance, and finally causing the increase in blood flow resistance.
Studies have also confirmed that the levels of inflammatory factors (e.g., C3, C4, IgG, IgE, IgA) in visceral venous wall in portal hypertension are significantly higher than that in the normal controls[11], suggesting that there are obvious immune inflammations in the vessels of portal system in the patients with portal hypertension, and the immune complex deposit and immune response may participate in the process of vasculopathy in portal hypertension.
In some studies, immunohistochemistry and double labeling immunofluorescence combined with laser scanning confocal microscopy are applied to detect the expressions of eNOS, ET-1, PKC, NF-kB in splenic vein of the patients with portal hypertension and portal endothelial cells of mouse portal hypertension model in order to investigate the relationship between the changes in the expression of eNOS and ET-1 in vascular endothelial cells in portal hypertension and signaling molecules PKC and NF-kB. The results showed that in the splenic vein of patients with portal hypertension and portal endothelial cells of mouse portal hypertension model, the expression of PKC was positive or strong positive, while in the controls, the expression of PKC was negative or weak positive[12]. Double labeling immunofluorescence combined with laser scanning confocal microscopy revealed that eNOS, ET-1, and NF-kB were mainly expressed in vascular endothelia and the fluorescent signaling intensity of them in the splenic vein of patients with portal hypertension and portal endothelial cells of mouse portal hypertension model was stronger than controls, suggesting that the increases of ET-1 and NO in venous blood was attributed to the increased synthesis from endothelial cells in portal system. The increased NO production in endothelial cells is related to the upregulation of eNOS expression. In portal hypertension, the mechanical stress signaling pathway in vascular endothelial cells is activated, which is responsible for the upregulation of ET-1 and eNOS expression in endothelial cells.
Recent studies showed that during the development of vasculopathy, endothelial cells and vascular smooth muscle cells are major effector cells. Various traumatic factors, such as shear stress, hydrostatic pressure, endotoxin, cytokines, growth factors, and inflammatory mediators, can result in injury and detachment of endothelial cells, leading to the bareness of collagenous fibers and aggregation of platelets. Endothelial cells express adhesion molecules (e.g., ICAM-1), release chemotactic factors (e.g., MCP-1)[13] and complement fragment C5a after complement activation[14]. As chemotactic factors, they can promote the mononuclear cells to bind to endothelial cells, translocating through endothelial cells to subintima, also enhance the migration of vascular smooth muscle cells to subintima and the phenotypic shift. The migration to subintima and proliferation of vascular smooth muscle cells and synthesized large quantities of extracellular matrix depositing in vascular wall to form neointima can induce remodeling of vessels before vasculopathy occurs. During this process, the interactive regulation between endothelial cells and vascular smooth muscle cells runs through the development of vasculopathy. Endothelial cells are the permissive cells to various stimulators, while vascular smooth muscle cells are the effector cells. This is a repair response of vessels to varied injures, at the same time, a pathological process of vasculopathy.
Gene regulation
c-fos is a message transferring factor. Dzau et al[15] have found that after vascular smooth muscle cells receive message, nuclear c-fos is expressed, the synthesis of DNA and mRNA is increased and cells proliferate. In the patients with hepatic cirrhosis, the expression of c-fos in splenic vein is increased, indicating that the proliferation, migration, and phenotypic shift of smooth muscle cells are related to the activation of oncogenes. The signaling pathway of c-fos that connects the mechanical signaling with the expression of proliferating gene is the biological basis of vascular remodelization.
Some suppressive oncogenes, such as p53, the effective regulatory factor for apoptosis, can promote the apoptosis of vascular smooth muscle cells, while prokaryotic genes, such as c-myc and bcl-2, can inhibit the apoptosis of these cells[16,17]. In patients with portal hypertension, the bax gene is strongly expressed and takes part in the regulation of apoptosis of smooth muscle cells in portal hypertension, indicating that the process of vasculopathy in portal hypertension involves gene modulation.
MAPK pathway is one of the important signaling conductive pathways modulating cellular proliferation, differentiation, and apoptosis[18,19]. Many extracellular stimulators, such as ultraviolet radiation[20], oxygen stress[21,22], cytokines[23,24], inflammatory mediators[25-27], can stimulate the cells to upregulate the expression and activation of MAPK. In an experiment using the specific antibody directed against Tyr-phosphorylated site at ERK1/2204, the results revealed that in portal hypertension, the expressions of phosphorylated ERK1/2 and its downstream factor c-fos were increased in vascular wall of splenic vein, indicating that the activation of ERK1/2/c-fos signaling transduction pathway might contribute to the development of vasculopathy in portal hypertension.
Collagen, an important vascular extracellular matrix protein, can retain the flexibility and intensity of vascular wall. In normal human vascular wall, the predominant collagen subtypes are types I, III, and V[28]. When vascular endothelia are damaged, collagenous fibers, as a strong promoter factor, can promote the aggregation of platelets. During the process of intervention-induced vascular injury and atherogenesis[29,30], the synthesis of vascular wall types I and III collagen and procollagen is increased. Types I and III collagen and procollagen are all the components of extracellular matrix, synthesized and secreted mainly by vascular smooth muscle cells in vasculopathy. A study has shown that a slight change in the constitutional ratio of types I and III collagen can trigger atherogenesis[12]. In the study on vasculopathy in portal hypertension, it was found that in the gastric coronary venous wall, the synthesis of connectin and laminin was increased, suggesting that extracellular matrix might play an important role in the pathogenesis of vasculopathy in portal hypertension. In several studies, RT-PCR was used to detect the expression of types I and III procollagen in portal hypertensive vasculopathy to investigate the mechanism of vasculopathy. The results showed that in portal hypertension, the expression of type III procollagen mRNA in splenic venous wall was 5-6 times higher than that in the patients with non-portal hypertension (P<0.01), while there was no significant difference in the expression of type I procollagen mRNA between them. It demonstrated that in the development of vasculopathy in portal hypertension, type III procollagen, depositing in vascular wall when extracellular matrical components are greatly expressed, is one of the important factors promoting the remodelization of splenic veins.
Vasoactive substances
Vasoactive substances act on vascular smooth muscle cells through paracrine and autocrine system. Immunohistoche-mistry confirmed that in portal hypertension, the activities of iNOS, P substance, and VIP in visceral arterial wall were increased[8]. Among them, the expression of iNOS in vascular smooth muscle cells of splenic vein was increased, indicating that the production of NO was increased correspondingly. NO, a stronger vascular diastolic factor, can attenuate the response of vessels to endogenous systolic active substances, which is contributed to the activation of soluble guanylate cyclase in vascular smooth muscle cells by NO, leading to the increased production of cGMP, and intracellular Ca2+, diastolization of smooth muscles, dilation of vessels, and decreased resistance. However, in hepatic cirrhosis-induced portal hypertension, the expression and activity of eNOS is reduced[31]. Transfection of eNOS[32] or heterogenic nNOS[33] genes by genetic techniques[3] could significantly reduce the animal portal pressure. By using PCR assay, Morales-Ruiz et al[34] confirmed the expression of iNOS mRNA in thoracic aorta, abdominal aorta, and mesenteric arteries. The studies by Suga et al[35] proved that the diastolic effect of mesenteric arteries to vasodilators in response was stronger than that of portal system. It is suggested that the over-release of NO may play an important role in the pathogenesis of visceral vascular low systolization and diastolization in portal hypertension[36].
Endothelin (ET) is the strongest vasoconstrictive polypeptide, crucially regulating the hepatic microcirculation and portal pressure. By using RT-PCR and RIA assays, Tieche et al, reported that in the mouse model of hepatic cirrhosis caused by bile duct ligation, the expression of ET gene was increased 6 times at 3 d after ligation and 30 times at 28 d after ligation. Moreover, it was directly correlated with the activation of hepatic stellate cells (HSCs) and the increased portal pressure. The increased expression and activity of ET gene were more obvious in portal vein and hepatic microcirculation than that in visceral arteries, and its systolic effect on veins was significantly stronger than that on arteries[37], suggesting that ET and NO are two important antagonists against portal hypertension.
AngII is an endogenous eight peptide and induces the increase in the targeting cellular Ca2+ concentration by binding to the specific receptor on the membrane of targeting cells to form a receptor-ligand compound. The increased Ca2+, as a second messenger, can produce the intensive effects of vasoconstriction and promote smooth muscle cell proliferation by different cascade reactions. In hepatic cirrhosis-induced portal hypertension, the vasodilator substances, such as NO and PGT2, can directly or indirectly reduce the expression of AngII receptors (AT1 and AT2) in vascular endothelial cells and decrease their affinity to AngII on the surface of smooth muscle cells. Both receptors in combination with the phenotypic changes of obviously hypertrophic or hyperplastic vascular smooth muscle cells can influence the responses of vessels to AngII[38].
TGF-b plays a crucial role in regulating the deposition of external vascular matrix[39], promotes the transcription of types I and III procollagen, and contributes to fibrosis in liver and other organs noticeably. In animal models of hepatic cirrhosis and patients with chronic hepatitis, the increased TGF-b concentration was associated with the deposition of internal vascular matrix in the targeting organs. In HSCs, TGF-b could promote the synthesis of collagen[40] Administration of TGF-b antibody or antagonists of TGF-b receptors can inhibit the synthesis of collagen. In contrast, the addition of exogenous TGF-b can increase the synthesis of collagen in smooth muscle cells[41,42]. TGF-b has many functions and exerts its biological effects via TGF-b receptors. TGF-b binds with its receptors before phosphor-ylation of the Smad protein and then enters into nuclei to regulate the transcription of proteins[43]. Also, TGF-b can increase the synthesis of extracellular matrix by activating Ras, Raf-1, and MEK in sequence[44,45].
NF-kB, as an important transcriptional regulatory factor, plays a key role in vasculopathy in portal hypertension[46]. After activation, the inhibition subunit I-kB of NF-kB is released into the nuclei to promote the transcription of fibrosis-associated genes via the JNK, AP-1 pathway. NK-kB is also the transcription of intercellular adhesion molecule-1 and vascular cell adhesion molecule-1[47]. It is known that besides the increased deposition of external vascular collagen for the vascular remodeling, the main factors for angiosclerosis are extracellular matrix and the binding sites of smooth muscle cells. During this process, adhesion molecules take part in regulating the binding of smooth muscles with extracellular matrix, promoting the development of vasculopathy.
Activator protein-1 (AP-1) binds with the AP-1 binding sites at the VEGF gene promoter that can promote transcription of VEGF[48]. VEGF acts through various pathways. Firstly, VEGF, the known strongest factor enhancing the vascular permeability, can increase the permeability of vascular wall[49]. VEGF functions through its receptors FLt-1 and FLk-1. In hepatic fibrosis, visceral vessels mainly express FLt-1, by which VEGF can promote the cell proliferation and collagen synthesis[50]. Secondly, VEGF possesses the chemotaxis to mononuclear macrophages and increases the adhesion of macrophages. The increased vascular permeability and the entry of mononuclear cells and macrophages into vascular intima result in the release of inflammatory factors, which can promote the smooth muscle cells proliferation and increase the synthesis of collagen[51]. VEGF can interact with basic cellular growth factor, TGF-b and hyaluronic acid to coordinately promote the proliferation of smooth muscle cells and collagen synthesis.
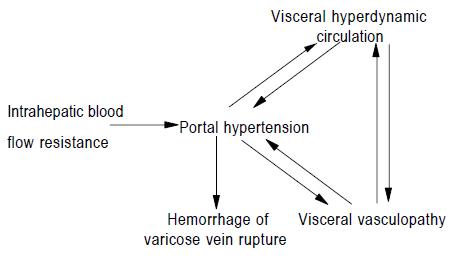
To sum up, the pathological changes of vasculopathy in portal hypertension include arterialized remodeling of visceral veins, the intimal injury of visceral veins and the destruction of contractile structure in visceral arterial wall. The mechanism of vasculopathy in portal hypertension may be attributed to the changes of hemodynamics in portal system, immune response, gene modulation, vasoactive substances, and intrahepatic blood flow resistance. On the one hand, portal hypertension can cause visceral hyperdynamic circulation and the development and progression of visceral vasculopathy. The visceral vasculopathy includes the destruction of contractile structure in visceral arterial wall and the remodelization of visceral veins. Vasculopathy results from the increased portal pressure and blood flow. On the other hand, visceral vasculopathy can promote the development and progression of portal hypertension and visceral hyperdynamic circulation in turn. The aforementioned three factors interact in the pathogenesis of hepatic cirrhosis-induced portal hypertension and are involved in hemorrhage of varicose vein rupture. The interaction between them is demonstrated as follows[6]:
Math 1