Published online Oct 14, 2005. doi: 10.3748/wjg.v11.i38.5997
Revised: February 13, 2005
Accepted: February 18, 2005
Published online: October 14, 2005
AIM: To examine the effect of acute infection caused by herpesvirus (pseudorabies virus, PRV) on pancreatic ductal secretion.
METHODS: The virulent Ba-DupGreen (BDG) and non-virulent Ka-RREp0lacgfp (KEG) genetically modified strains of PRV were used in this study and both of them contain the gene for green fluorescent protein (GFP). Small intra/interlobular ducts were infected with BDG virus (107 PFU/mL for 6 h) or with KEG virus (1010 PFU/mL for 6 h), while non-infected ducts were incubated only with the culture media. The ducts were then cultured for a further 18 h. The rate of HCO3- secretion [base efflux -J(B-)] was determined from the buffering capacity of the cells and the initial rate of intracellular acidification (1) after sudden blockage of basolateral base loaders with dihydro-4,4-diisothiocyanatostilbene-2,2-disulfonic acid (500 mmol/L) and amiloride (200 mmol/L), and (2) after alkali loading the ducts by exposure to NH4Cl. All the experiments were performed in HCO3--buffered Ringer solution at 37°C (n = 5 ducts for each experimental condition). Viral structural proteins were visualized by immunohistochemistry. Virally-encoded GFP and immunofluorescence signals were recorded by a confocal laser scanning microscope.
RESULTS: The BDG virus infected the majority of accessible cells of the duct as judged by the appearance of GFP and viral antigens in the ductal cells. KEG virus caused a similarly high efficiency of infection. After blockage of basolateral base loaders, BDG infection significantly elevated -J(B-) 24 h after the infection, compared to the non-infected group. However, KEG infection did not modify -J(B-). After alkali loading the ducts, -J(B-) was significantly elevated in the BDG group compared to the control group 24 h after the infection. As we found with the inhibitor stop method, no change was observed in the group KEG compared to the non-infected group.
CONCLUSION: Incubation with the BDG or KEG strains of PRV results in an effective infection of ductal epithelial cells. The BDG strain of PRV, which is able to initiate a lytic viral cycle, stimulates HCO3- secretion in guinea pig pancreatic duct by about four- to fivefold, 24 h after the infection. However, the KEG strain of PRV, which can infect, but fails to replicate, has no effect on HCO3- secretion. We suggest that this response of pancreatic ducts to virulent PRV infection may represent a defense mechanism against invasive pathogens to avoid pancreatic injury.
- Citation: Hegyi P, Ördög B, Jr ZR, Takács T, Lonovics J, Szabolcs A, Sári R, Tóth A, Papp JG, Kovács AVMK, Gray MA, Argent BE, Boldogköi Z. Effect of herpesvirus infection on pancreatic duct cell secretion. World J Gastroenterol 2005; 11(38): 5997-6002
- URL: https://www.wjgnet.com/1007-9327/full/v11/i38/5997.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i38.5997
Pseudorabies virus (PRV) is an ?-herpesvirus closely related to the herpes simplex virus (HSV), a well-known human pathogen[1,2]. The natural host of PRV is the pig, but it has a very wide host range including several mammalian families, such as carnivores, ungulates and rodents, but humans are resistant to PRV infection[1]. PRV infection is a multistep process initiated by the receptor-mediated attachment of the virus to the cell surface. Upon entering the cells, the PRV nucleocapsid approaches the nuclear membrane and the viral DNA is released into the nuclei by a poorly understood mechanism. The lytic cycle of the virus is controlled by a transcriptional cascade mechanism[2].
Acute pancreatitis is most commonly associated with biliary stone and alcoholism. Other causes, including infections, drugs, lipid abnormalities, congenital anomalies, trauma, tumors, and idiopathic form, account for at least 10-20% of the total number of cases[3]. The incidence of pancreatitis may even be higher since the diagnosis of acute pancreatitis may go undetected in many patients. Several viruses have been reported to cause acute pancreatitis, including coxsackievirus B3[4] and B4[5] mumpsvirus[6] varicella[7] hepatitis A[8] and B[9] cytomegalovirus[10] varicella-zoster virus[11] and HSV[12].
We have previously shown that hypersecretion can be observed during the early phase of experimental edematous and necrotizing pancreatitis[13]. However, the source and the role of this hypersecretion are unknown. Either acinar cells and/or the ductal epithelium could be responsible for hypersecretion.
Our aim in this study was to examine the effect of acute infection caused by PRV on pancreatic ductal secretion.
Ba-Dup Green (BDG), a genetically modified replicating strain of PRV, was used in this study. The Bartha virus[14] which is an attenuated live vaccine strain of PRV, was used as the parental virus for the generation of BDG. Two copies of a genetically modified green fluorescent protein (GFP) gene-containing expression cassettes were inserted into the PRV antisense promoter region located in the inverted repeat segment of the virus[15,16]. Ka-EP0lacgfp (KEG) strain of PRV was used as a non-replicating control virus for these experiments. KEG was constructed by deleting the small subunit of ribonucleotide reductase[17] and the early protein 0 (EP0)[18] genes. An expression cassette containing a GFP and a lacZ gene was inserted in place of the EP0 gene[19]. These mutations render the virus incapable of replication in non-dividing cells[19].
Small intra/interlobular ducts were isolated from the pancreas of guinea pigs weighing 150-250 g. The guinea pig was humanely killed by cervical dislocation, the pancreas was removed and intra/interlobular ducts were isolated by enzymatic dissociation, microdissection and then cultured at 37 ? in 50 mL/L CO2[20]. Ducts were incubated in McCoy?-based culture medium containing BDG viruses at a dose of 107 PFU/mL for 6 h or KEG virus (1010 PFU/mL for 6 h), while the non-infected ducts were exposed to culture media only. The ducts were then cultured for a further 18 h as described earlier[20] during which time the ducts seal to form a closed sac that swells due to accumulation of ions and water secretions within the duct lumen[20].
The immunofluorescent detection of structural viral proteins was performed as follows. Isolated pancreatic ducts were fixed in40 g/L paraformaldehyde in PBS, pH 7.4 for 1 h at room temperature. Following three rinses in PBS, the ducts were blocked with 10 g/L bovine serum albumin and 1 mL/L Triton X-100 in PBS for 1 h at room temperature. Thereafter, a rabbit polyclonal antiviral antibody (Affinity Bioreagents, Golden, USA) diluted at 1:500 (v/v) in blocking solution was applied for 24 h at 4°C. After being washed with PBS, the ducts were incubated with an anti-rabbit antibody conjugated with a red light-emitting dye (Alexa Fluor 633; Molecular probes, USA) diluted at 1:1 000 (v/v) in a blocking solution for 24 h at 4°C. After further washing in PBS, the ducts were mounted on slides using Aqua-Poly/Mount (Polysciences Inc., Niles, USA). The specificity of immunohistochemical procedure was assessed by simultaneous staining of non-infected ducts. No immunohistochemical labeling was observed in control experiments.
For monitoring virally expressed GFP and immunofluorescence signals, fluorescent images and optical sections of the infected ducts were recorded by a confocal laser scanning microscope (Zeiss, LSM 400).
Cultured ducts were attached, using Cell-Tak, to a coverslip (24 mmol/L) forming the base of a perfusion chamber mounted on a Nikon Diaphot microscope (Nikon UK Ltd, Budapest, Hungary). The ducts were bathed in the standard Hepes solution at 37°C and loaded with the pH-sensitive fluorescent dye 2,7-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein (BCECF) by exposure to 2 mmol/L BCECF-AM for 20-30 min. After loading, the ducts were continuously perfused with solutions at a rate of 4-5 mL/min. Intracellular pH (pHi) was measured using a microspectrofl-uorimeter system (Cairn, Kent, UK). A small area of 5-10 cells was excited with light at wavelengths of 490 and 440 nm, and the 490/440 fluorescence emission ratio was measured at 535 nm. Four pHi measurements were obtained per second. In situ calibration of the fluorescence signal was performed using the high K+-nigericin technique[21,22]. During calibration, the ducts were bathed in high K+ HEPES solution and extracellular pH stepped between 5.95 and 8.46.
The intrinsic buffering capacity (bi) of duct cells was estimated according to the NH4+ pre-pulse technique[23,24]. bi refers to the ability of intrinsic cellular components (excluding HCO3-/CO2) to buffer changes of pHi. Briefly, pancreatic duct cells were exposed to various concentrations of NH4Cl, while Na+ and HCO3- were omitted from the solution in order to block the Na+-dependent pH regulatory mechanisms. bi was estimated by the Henderson-Hasselbach equation. The total buffering capacity (btotal) was calculated from: btotal = bi+bHCO3- = bi+2.3×HCO3-]i, where bHCO3- is the buffering capacity of the HCO3-/CO2 system and [HCO3-]i is the intracellular HCO3- concentration[24].
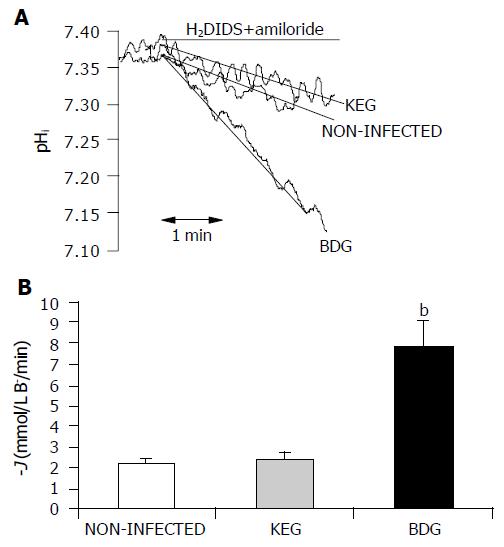
Inhibitor stop method Exposing the ducts to dihydro-4,4-diisothiocyanatostilbene-2,2-disulfonic acid (H2DIDS, 0.5 mmol/L) and amiloride (0.2 mmol/L) for 5 min caused a marked acidification of pHi (Figure 2A). This acidification occurred due to inhibition of the basolateral Na+/HCO3- co-transporters and Na+/H+ exchangers, which normally act to transport HCO3- into the duct cell from the blood[25,26]. The rate of pHi acidification after the exposure to H2DIDS and amiloride could reflect the intracellular buffering capacity and the rate at which HCO3- effluxes (i.e., secreted) across the apical membrane via Cl-/HCO3-exchangers and CFTR channels[24,25].
The initial rate of intracellular acidification (dpH/dt), over the first 60 s of exposure to amiloride and H2DIDS, was calculated by linear regression analysis using 240 data points (four pHi measurements per second)[24].
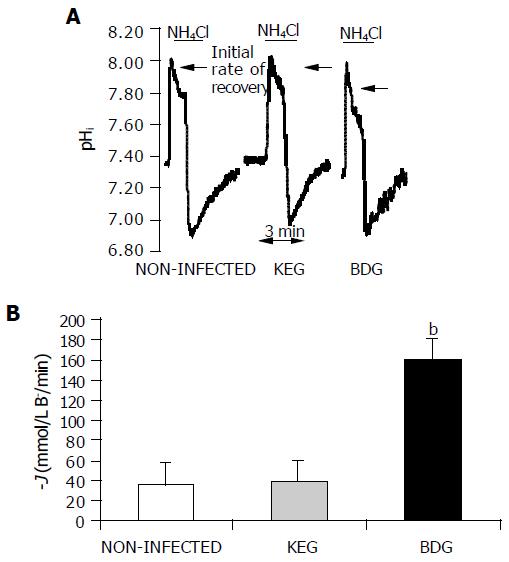
Alkali load method Exposing the ducts to 20 mol/L NH4Cl caused an alkalization of pHi due to the rapid influx of NH3 into cells (Figure 3A). Recently, we demonstrated that the recovery of pHi under these conditions was dependent on the presence of HCO3- in the bathing solution, suggesting that it results from HCO3- efflux (i.e., secretion) out of the duct cells[24]. In the present study, the initial rate of recovery from alkalosis (dpH/dt) over the first 30 s (120 pHi measurements) in the continued presence of NH4Cl was calculated as described previously[24].
The rates of pHi change measured in these inhibitors stopped and alkali load experiments were converted to transmembrane base flux J(B-) using the equation: J(B-)= dpH/dt×®total. We denoted base influx as J(B-) and base efflux (secretion) as -J(B-).
The standard Hepes-buffered solution contained (mmol/L) 130 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, 10 D-glucose, and 10 Na-Hepes. The high K+ Hepes-buffered solution contained (mmol/L) 130 KCl, 5 NaCl, 1 CaCl2, 1 MgCl2, 10 D-glucose, and 10 Na-Hepes. Hepes-buffered solutions were gassed with 100% O2 and their pH was set to 7.4 at 37°C with HCl. The standard HCO3--buffered solution contained (mmol/L) 115 NaCl, 25 NaHCO3, 5 KCl, 1 CaCl2, 1 MgCl2, and 10 D-glucose. The ammonia pulse solution contained (mmol/L) 95 NaCl, 20 NH4Cl, 25 NaHCO3, 5 KCl, 1 CaCl2, 1 MgCl2, and 10 D-glucose. HCO3--buffered solutions were gassed with 95% O2/50 mL/L CO2 to set pH to 7.4 at 37°C.
Chromatographically pure collagenase was obtained from Worthington (Lakewood, NJ, USA), culture media from Sigma (Budapest, Hungary). Nigericin (Sigma, Budapest, Hungary) was dissolved in absolute ethanol, H2DIDS (from Molecular Probes,Eugene, OR, USA) and amiloride in dimethyl sulfoxide (DMSO). CellTak was obtained from Becton Dickinson Labware (Bedford, MA, USA). BCECF-AM was obtained from Molecular Probes (Eugene, OR, USA) and was made up as a 2 mmol/L stock solution using DMSO. The 10-fold concentrated PBS stock solution contained (g/L) 80 NaCl, 2 KCl, 2.4 NaH2PO4, 14.4 Na2HPO4 (pH 7.2). Chemicals were obtained from Merck (Darmstadt, Germany). Blocking solution contained 10 g/L bovine serum albumin from Sigma (Budapest, Hungary) and 1 mL/L scintillation grade Triton X-100 (BDH Chemicals, Poole, UK).
Results were expressed as mean±SE (n = 5 ducts). Statistical analyses were performed using ANOVA. P<0.05 was accepted as statistically significant.
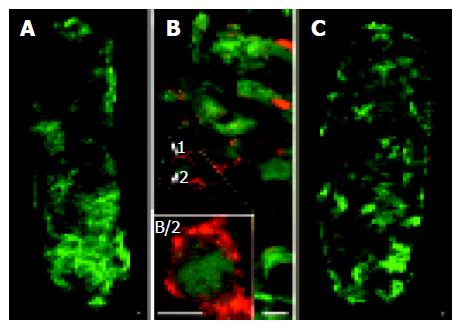
Two GFP-expressing cassettes were inserted into the PRV genome[15] enabling virus infection to be visualized in both living and fixed cells. Monitoring of GFP expression of BDG was utilized to assess the extent of viral infection of ductal epithelial cells. We observed that BDG virus practically infected all accessible cells of the duct when used at a titer of 107 PFU/mL after 24 h (Figure 1A). We used a higher concentration (1010 PFU/mL) of KEG virus and found a similarly high efficiency of infection (Figure 1C).
BDG is a virulent strain; therefore, virus uptake by cells results in the immediate initiation of the lytic viral cycle and the unavoidable death of the infected cells. Productive virus infection was detected in the BDG group by labeling the structural viral proteins by means of immunohistochemistry. The GFP gene was placed under the control of the constitutive human cytomegalovirus immediate early promoter, which conferred a very early expression of the reporter gene. In contrast, viral structural proteins, which are recognized by the anti-PRV antibodies, appeared at a later stage of viral infection[15]; therefore, a shift between the appearance of GFP and immunofluorescence could be observed (Figure 1B). In contrast, in the KEG group, we did not observe any cytopathic effects and could not detect viral antigens. However, GFP expression indicated the presence of the virus within the infected cells (Figure 1C).
Exposing the ducts to 0.5 mmol/L H2DIDS and 0.2 mmol/L amiloride caused an acidification of pHi (Figure 2A), due to inhibition of the basolateral Na+/HCO3- co-transporters and Na+/H+ exchangers. The effect of H2DIDS and amiloride was reversible, removal of the inhibitors caused pHi to return to the control value. In these series of experiments, we tested whether virus infection affected the net HCO3- secretion of pancreatic duct cells. Base efflux was significantly elevated in the BDG group compared to the non-infected ducts, 24 h after the infection (7.841.24 mmol/L B-/min vs 2.20.18 mmol/L B-/min, respectively;n = 5). However, no change was observed in the KEG group (2.40.32 mmol/L B-/min; n = 5) compared to the non-infected ducts. These data are summarized in Figure 2B.
Exposure of duct cells to 20 mmol/L NH4Cl induced an immediate rise in pHi due to the rapid entry of NH3 into the duct cells (Figure 3A). In this series of experiments, base efflux was significantly elevated in the BDG group compared to the control group 24 h after the infection (160.0419.16 mmol/L B-/min vs 36.371.08 mmol/L B-/min, respectively; n = 5). However, as we found with the inhibitor stop method, no change was observed in the KEG group (39.343.49 mmol/L B-/min, n = 5) compared to the non-infected ducts (Figure 3B).
Using the ammonium pulse technique, we also tested whether PRV affected the ability of duct cells to recover from an acid load following removal of NH4Cl from the superfusate. The transporters most likely to be involved in this process are the Na+/HCO3- co-transporter, the Na+/H+ exchanger and the H+ pump located on the basolateral membrane of the duct cells. No significant change was observed between the PRV infected and non-infected groups (Figure 3A, n = 5).
Though the broad spectrum of etiological factors is involved in acute pancreatitis, the pathophysiology of the disease is less understood. Most investigators believe that acute pancreatitis results from an early intra-acinar cell activation of zymogens[26]. Following this early activation, a trypsin cascade occurs in the gland leading to the auto-digestion of acinar cells[26]. However, a possible pathophysiological role of the ductal epithelium has not been investigated. The permeability of the pancreatic ductal epithelium to HCO3- and Cl- is increased by exposure to various bile salts at concentrations within the range normally found in the duodenum[27]. Moreover, E. coli-infected bile causes further increases of the permeability of the ductal epithelium to HCO3- and Cl-[27]. Ethanol could induce fluid hypersecretion from guinea pig pancreatic duct cells. Low concentrations of ethanol directly augment pancreatic ductal fluid secretion stimulated by physiological and pharmacological concentrations of secretin (cAMP pathway) and via Ca2+ mobilization[28]. The effects of other etiologic factors for acute pancreatitis on pancreatic ductal HCO3- secretion have not been characterized as yet.
Viruses can alter ion transport by epithelial cells. Kunzelmann et al[29] reported that parainfluenza virus I (Sendai virus) produces rapid changes in ion transport across tracheal epithelium. The Sendai virus, at concentrations observed during respiratory infections, activates Cl- secretion and inhibits Na+ absorption[29] by triggering the release of ATP, which then acts on apical P2Y receptors to produce changes in ion transport[29]. Bacterial infection (e.g., Pseudomonas aeruginosa orStaphylococcus aureus) triggers mucus and interleukin production[30,31]. Mucus clearance is a primary innate defense mechanism for mammalian airways[32].
In this study, we developed a model to investigate the effect of acute infection with a herpes virus (PRV) on the pancreatic ductal epithelium. Incubating BDG at a dose of 107 PFU/mL for 6 h resulted in the infection of the majority of accessible epithelial cells within the duct. As expected, the virulent PRV strain resulted in a productive infection of epithelial cells, indicated by the appearance of viral antigens. We used two different measures of HCO3- secretion (inhibitor stop and alkali load methods) to study the effect of acute PRV infection on pancreatic ducts. Both methods showed that BDG infection stimulated HCO3- secretion in guinea pig pancreatic duct by about four or fivefold. However, BDG had no effect on the pHi recovery after an acid load, suggesting that neither the basolateral Na+/HCO3- co-transporter nor the basolateral Na+/H+ exchanger is involved in the hypersecretory effect. We have previously reported that pancreatic hypersecretion is observed during the early phase of acute necrotizing pancreatitis[33]. Furthermore, the early phase hypersecretion is accompanied with a simultaneous decrease in protein output[34] suggesting that the pancreatic ducts are at least in part involved in the change of secretory pattern. In our study, the genetically engineered control virus (KEG) did not evoke hypersecretion in the ductal cells, indicating that the presence of the virus in the cell is not enough to trigger hypersecretion. Hypersecretion was only induced by the BDG virus, which is able to initiate a lytic viral cycle.
Our finding that BDG stimulates HCO3- secretion from pancreatic ducts may represent a defense mechanism against invasive pathogens in order to avoid pancreatic injury. We speculate that the stimulated secretion can wash out activated enzymes, viruses and other toxic factors from the pancreas. Moreover, the high efficiency of PRV infection in the pancreatic ductal epithelium may open the possibility for gene transfer and gene therapy of the duct cells using non-replicating or conditionally replicating PRV variants (KEG)[2,19].
We would like to thank Dr. J?os Szabad for his helpful discussions.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
Co-first-authors: Péter Hegyi and Balázs Ördög
| 1. | Boldogkoi Z, Bratincsak A, Fodor I. Evaluation of pseudorabies virus as a gene transfer vector and an oncolytic agent for human tumor cells. Anticancer Res. 2002;22:2153-2159. [PubMed] |
| 2. | Boldogköi Z, Nógrádi A. Gene and cancer therapy--pseudorabies virus: a novel research and therapeutic tool? Curr Gene Ther. 2003;3:155-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Steer ML. Etiology and pathophysiology of acute pan-creatitis. In: The Exocrine Pancreas: Biology, Pathobiology and Diseases. Edited by Go VLW, Dimagno EP, Gardner JD, Lebenthal E, Reber HA, Scheele GA. Raven Press New York 1993; 581-593. |
| 4. | Vuorinen T, Kallajoki M, Hyypiä T, Vainionpää R. Coxsackievirus B3-induced acute pancreatitis: analysis of histopathological and viral parameters in a mouse model. Br J Exp Pathol. 1989;70:395-403. [PubMed] |
| 5. | Vella C, Brown CL, McCarthy DA. Coxsackievirus B4 infection of the mouse pancreas: acute and persistent infection. J Gen Virol. 1992;73:1387-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Imrie CW, Ferguson JC, Sommerville RG. Coxsackie and mumpsvirus infection in a prospective study of acute pancreatitis. Gut. 1977;18:53-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 47] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Kirschner S, Raufman JP. Varicella pancreatitis complicated by pancreatic pseudocyst and duodenal obstruction. Dig Dis Sci. 1988;33:1192-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Khanna S, Vij JC. Severe acute pancreatitis due to hepatitis A virus infection in a patient of acute viral hepatitis. Trop Gastroenterol. 2003;24:25-26. [PubMed] |
| 9. | de Oliveira LC, Rezende PB, Ferreira AL, de Freitas AA, de Carvalho AM, Guedes CA, Costa WO. Concurrent acute hepatitis and pancreatitis associated with hepatitis B virus: case report. Pancreas. 1998;16:559-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Wilcox CM, Forsmark CE, Grendell JH, Darragh TM, Cello JP. Cytomegalovirus-associated acute pancreatic disease in patients with acquired immunodeficiency syndrome. Report of two patients. Gastroenterology. 1990;99:263-267. [PubMed] |
| 11. | Pulik M, Teillet F, Teillet-Thiebaud F, Lionnet F, Genet P, Petitdidier C. Varicella-zoster virus pancreatitis in hematologic diseases. Ann Med Interne (Paris). 1995;146:292-294. [PubMed] |
| 12. | Shintaku M, Umehara Y, Iwaisako K, Tahara M, Adachi Y. Herpes simplex pancreatitis. Arch Pathol Lab Med. 2003;127:231-234. [PubMed] |
| 13. | Hegyi P, Czako L, Takacs T, Szilvassy Z, Lonovics J. Pancreatic secretory responses in L-arginine-induced pancreatitis: comparison of diabetic and nondiabetic rats. Pancreas. 1999;19:167-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Bartha A. Experimental reduction of virulence of Aujeszky's disease (in Hungarian). Magy Allatorv Lapja. 1961;16:42-45. |
| 15. | Boldogköi Z, Reichart A, Tóth IE, Sik A, Erdélyi F, Medveczky I, Llorens-Cortes C, Palkovits M, Lenkei Z. Construction of recombinant pseudorabies viruses optimized for labeling and neurochemical characterization of neural circuitry. Brain Res Mol Brain Res. 2002;109:105-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Boldogköi Z, Sík A, Dénes A, Reichart A, Toldi J, Gerendai I, Kovács KJ, Palkovits M. Novel tracing paradigms--genetically engineered herpesviruses as tools for mapping functional circuits within the CNS: present status and future prospects. Prog Neurobiol. 2004;72:417-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Boldogkói Z, Medveczky I, Glávits R, Braun A, Fodor I. In vivo studies on Aujeszky's disease virus mutants. Acta Microbiol Immunol Hung. 1996;43:307-318. [PubMed] |
| 18. | Boldogköi Z, Braun A, Fodor I. Replication and virulence of early protein 0 and long latency transcript deficient mutants of the Aujeszky's disease (pseudorabies) virus. Microbes Infect. 2000;2:1321-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Boldogköi Z, Szabó A, Vrbová G, Nógrádi A. Pseudorabies virus-based gene delivery to rat embryonic spinal cord grafts. Hum Gene Ther. 2002;13:719-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Argent BE, Arkle S, Cullen MJ, Green R. Morphological, biochemical and secretory studies on rat pancreatic ducts maintained in tissue culture. Q J Exp Physiol. 1986;71:633-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Thomas JA, Buchsbaum RN, Zimniak A, Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979;18:2210-2218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1591] [Cited by in RCA: 1715] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 22. | Hegyi P, Rakonczay Z, Gray MA, Argent BE. Measurement of intracellular pH in pancreatic duct cells: a new method for calibrating the fluorescence data. Pancreas. 2004;28:427-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Weintraub WH, Machen TE. pH regulation in hepatoma cells: roles for Na-H exchange, Cl-HCO3 exchange, and Na-HCO3 cotransport. Am J Physiol. 1989;257:G317-G327. [PubMed] |
| 24. | Hegyi P, Gray MA, Argent BE. Substance P inhibits bicarbonate secretion from guinea-pig pancreatic ducts by modulating an anion exchanger. Am J Physiol. 2003;285:C268-C276. |
| 25. | Szalmay G, Varga G, Kajiyama F, Yang XS, Lang TF, Case RM, Steward MC. Bicarbonate and fluid secretion evoked by cholecystokinin, bombesin and acetylcholine in isolated guinea-pig pancreatic ducts. J Physiol. 2001;535:795-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Lerch MM, Albrecht E, Ruthenbürger M, Mayerle J, Halangk W, Krüger B. Pathophysiology of alcohol-induced pancreatitis. Pancreas. 2003;27:291-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Reber HA, Mosley JG. The effect of bile salts on the pancreatic duct mucosal barrier. Br J Surg. 1980;67:59-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Yamamoto A, Ishiguro H, Ko SB, Suzuki A, Wang Y, Hamada H, Mizuno N, Kitagawa M, Hayakawa T, Naruse S. Ethanol induces fluid hypersecretion from guinea-pig pancreatic duct cells. J Physiol. 2003;551:917-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Kunzelmann K, König J, Sun J, Markovich D, King NJ, Karupiah G, Young JA, Cook DI. Acute effects of parainfluenza virus on epithelial electrolyte transport. J Biol Chem. 2004;279:48760-48766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | McNamara N, Khong A, McKemy D, Caterina M, Boyer J, Julius D, Basbaum C. ATP transduces signals from ASGM1, a glycolipid that functions as a bacterial receptor. Proc Natl Acad Sci USA. 2001;98:9086-9091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 107] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 31. | Ratner AJ, Bryan R, Weber A, Nguyen S, Barnes D, Pitt A, Gelber S, Cheung A, Prince A. Cystic fibrosis pathogens activate Ca2+-dependent mitogen-activated protein kinase signaling pathways in airway epithelial cells. J Biol Chem. 2001;276:19267-19275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 141] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 32. | Knowles MR, Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest. 2002;109:571-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 911] [Cited by in RCA: 780] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 33. | Hegyi P, Rakonczay Z, Sári R, Góg C, Lonovics J, Takács T, Czakó L. L-arginine-induced experimental pancreatitis. World J Gastroenterol. 2004;10:2003-2009. [PubMed] |
| 34. | Czakó L, Yamamoto M, Otsuki M. Exocrine pancreatic function in rats after acute pancreatitis. Pancreas. 1997;15:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |