Published online Sep 28, 2005. doi: 10.3748/wjg.v11.i36.5718
Revised: February 13, 2005
Accepted: February 18, 2005
Published online: September 28, 2005
AIM: To investigate the expression and distribution of HBV in the ovaries and ova.
METHODS: The immunohistochemistry method was used to detect the HBsAg and HBcAg in the ovaries of patients with chronic hepatitis B.
RESULTS: Expression of HBsAg in the ova, granular and interstitial cells of the ovaries was located in the cytomembrane and cytoplasm. Expression of HBcAg in the ova, granular, interstitial and endothelial cells of interstitial blood vessels of the ovaries was found in the cytomembrane, cytoplasm, and nuclei.
CONCLUSION: HBV can infect the ova at different stages of development and replicate in it.
- Citation: Ye F, Yue YF, Li SH, Chen TY, Zhang SL, Bai GQ, Liu M. Expression of HBsAg and HBcAg in the ovaries and ova of patients with chronic hepatitis B. World J Gastroenterol 2005; 11(36): 5718-5720
- URL: https://www.wjgnet.com/1007-9327/full/v11/i36/5718.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i36.5718
In Asian countries such as China, vertical infection plays a major role in transmitting HBV. The infected babies will eventually develop liver cirrhosis or hepatocellular carcinoma in their adulthood, and female babies will continue the cycle of vertical transmission to their offsprings[1-3]. Recent data indicate that intrauterine infection is one of the important routes of HBV vertical transmission, and the rate of intrauterine HBV infection is 10-44.4%[3,4]. Furthermore, studies have proved that most intrauterine infections can be prevented by inoculation with HBVac and HBIG in the perinatal stage, but 5-10% of the infections cannot be prevented by the combination of HBIG and HBVac[5,6]. The mechanism for this fact is controversial. Some researchers suspected that the infection of ova with HBV may be a factor in this mechanism[7]. But this was just a supposition, and little data exist to prove it.
To explore if HBV could infect the ovaries and ova, we detected the expression of HBsAg and HBcAg in the ovaries and ova of patients with chronic hepatitis B (CHB) by immunohistochemistry method.
Ovary tissues were obtained from 18 patients with CHB who received surgery for ovary disease at the First Hospital of Xi’an Jiaotong University in China. The specimens were collected after surgery. Serum samples from these patients were tested for different HBV markers including HBsAg, HBeAg, anti-HBc, anti-HBe (HBVM) using a commercially available ELISA kit (Sino- America Biological Technology, Inc., Beijing, China) prior to surgery. HBsAg was positive while HCV, HIV, HAV, and HEV were negative in 18 patients. All reactions were performed at the Immunohisto-chemistry Laboratory in Xi’an Jiaotong University.
Ovary tissues were fixed in 40 g/L formaldehyde, embedded with paraffin and cut into 4 µm thick sections. Mouse (McAb) against HBsAg (Dako, Denmark) and rabbit polyclonal antibodies against HBcAg (Baoxin, China) were used for immunohistochemical test. DAB staining kits were purchased from Wuhan BoShiDe Biological Engineering Ltd. Company.
HBsAg and HBcAg were detected by immunhisto-chemical staining using the avidin-biotin complex (ABC) method on the sections of ovarian tissues. In brief, formalin-fixed, paraffin-embedded sections were deparaffinized in xylene and passed through ethanol series. After the endogenous peroxidase activity was blocked, the sections were rinsed in 0.01 mol/L PBS. Non-specific binding was blocked by treatment with 5% normal serum for 30 min. Primary antibody was applied to the sections and incubated in a moist chamber overnight at 4°C. After the sections were washed in 0.01 mol/L PBS, second antibody was applied and sections were incubated for 30 min at 37°C in a moist chamber. After being washed, the sections were incubated with avidin-biotin-peroxidase complex for 30 min at 37°C in a moist chamber and washed again. The chromogen, 3-3-diaminobenzidine (DAB) was added to the sections for 10 min.
The sections of HBsAg and HBcAg positive livers from autopsy were used as positive control, and the sections of ovaries from HBVM negative women served as negative control. At the same time, PBS was used as blank control instead of the first antibody in immunohistochemical test. Dark brown yellow in cytoplasm, cytomembrane or nuclei was regarded as strongly positive, brown yellow as positive, and light brown yellow as weakly positive.
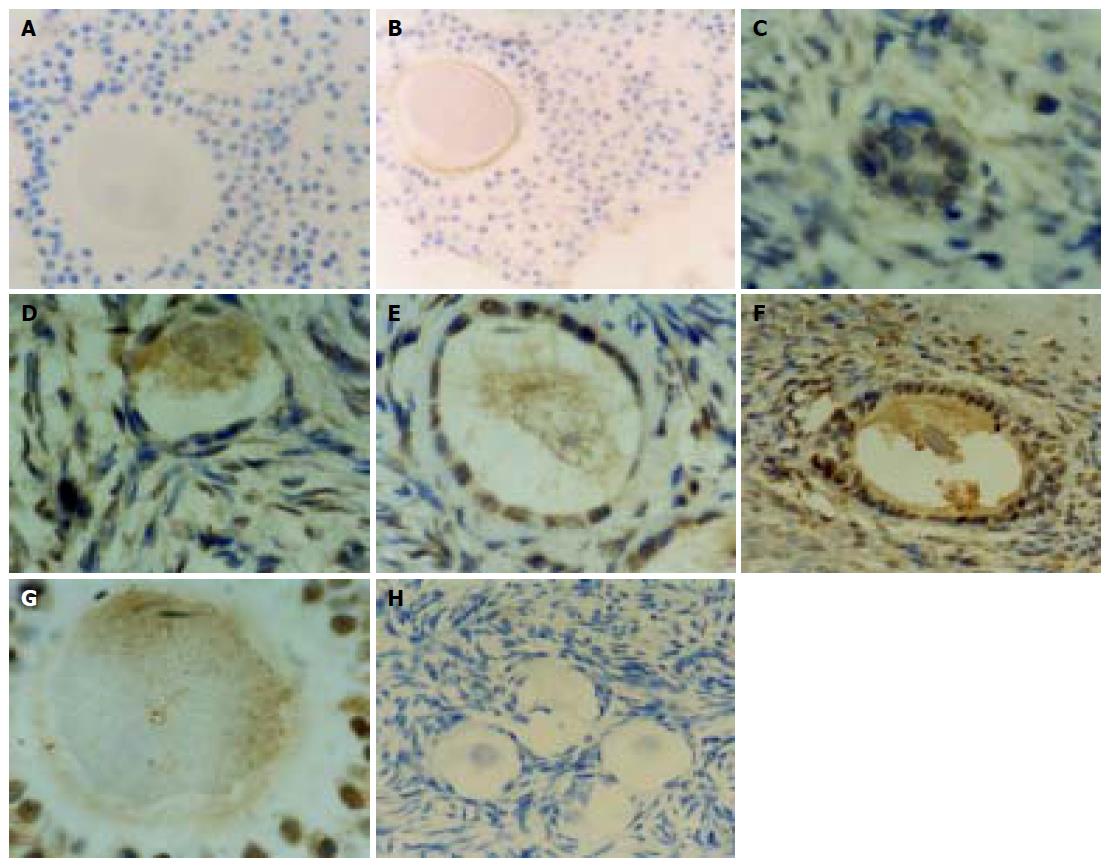
Expression of HBsAg in the ova and granular cells of the ovaries was located in the cytomembrane and cytoplasm. The positive rate was 11% (2/18). Negative control and blank control were negative. (Figures 1A and B) Expression of HBcAg in the ova, granular, interstitial and endothelial cells of interstitial blood vessels of the ovaries was found in the cytomembrane, cytoplasm, and nuclei. HBsAg and HBcAg in the ovaries were strongly positive. The positive rate was 45% (8/18). Negative control and blank control were negative. (Figures 1C-H).
Much attention has been paid to the effective prevention of vertical HBV transmission[1-4]. It was reported that intrauterine HBV is transmitted through HBV- infected placenta and HBV could enter the blood of infant through placental leak[8]. But this cannot explain why HBV can infect the embryo (46 d) . Chen et al[7] showed that HBV cannot infect the embryo through placenta. They believe there is another mechanism by which HBV infects the embryo.
Yu et al[9] proved that hemorrhagic fever virus exists in the ova of rodent. Bovine viral diarrhea virus can infect the ova of bovine[10]. Tagawa et al[11] found that duck HBV (DHBV) is expressed in yolk of duck with hepatitis B, and that DHBVDNA is present in liver of embryo after 6 d of incubation, suggesting DHBV could infect the egg of duck and replicate in it. Zhao et al[12] reported that DNA of TTV is found in the ovaries of CHB patients by hybridization in situ. Zhou et al[13] reported that HBVDNA is present in plasma of ova and in interstitial cells of the ovaries of a patient who died of serious hepatitis B. Taylor et al[14] have detected HBsAg in follicular fluid of CHB patients by immunohistochemistry method.
In this study, HBsAg and HBcAg were expressed in ova and granular cells of the ovaries. HBcAg was expressed in ova at different stages of development, suggesting that HBV can infect the ovaries and ova, and replicate in them. The fact suggests that HBV-infected ova may cause the vertical transmission of HBV, which cannot be prevented by inoculation of HBV vaccine and HBIG because the embryo is infected as zygote is formed.
In this study, the positive rate of HBsAg (11%) was lower than that of HBcAg (45%). We consider that the primary antibody against HBsAg is monoclonal antibody (McAb), but the primary antibody against HBcAg is polyclonal antibody. Since the binding epitope of McAb is less than that of polyclonal antibody, the positive rate of HBsAg is lower. On the other hand, in the ova, the synthetizing and expression of HBcAg by HBV may be higher than that of HBsAg in the ovaries. The amount of HBsAg is more than that of HBcAg in liver cells[15,16]. Of course, a little sample may be a reason.
In conclusion, HBV can infect the ovaries and ova and replicate in them. But how does HBV infect the ovaries and ova and whether HBV-infected ova results in HBV vertical transmission remain unknown.
The authors thank Xiao-Ge Zhao for his excellent technical assistance, Zhao-Yang Liu and Zi-Yun Shi for their surgical assistance.
Science Editor Wang XL Language Editor Elsevier HK
Co-first-authors: Feng Ye and Ya-Fei Yue
Co-correspondents: Ya-Fei Yue
| 1. | Li YX, Gao QW, Zhang YH, Guo Y, Li BW, Wang HX, Wang YL, Wang YM. [An investigation on the transmission routes and early diagnosis of intrauterine infection induced by hepatitis B virus]. Zhonghua Gan Zang Bing Za Zhi. 2004;12:18-20. [PubMed] |
| 2. | Yue YF, Jiang H, Shi L, Li LF, Xi BS, Yu YL, Chen GF. Study on the mechanism of intrauterine infection of hepatitis B virus. Zhonghua Fu Chan Ke Za Zhi. 2004;39:224-226. [PubMed] |
| 3. | Xu DZ, Yan YP, Choi BC, Xu JQ, Men K, Zhang JX, Liu ZH, Wang FS. Risk factors and mechanism of transplacental transmission of hepatitis B virus: a case-control study. J Med Virol. 2002;67:20-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 196] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 4. | Wang Z, Zhang J, Yang H, Li X, Wen S, Guo Y, Sun J, Hou J. Quantitative analysis of HBV DNA level and HBeAg titer in hepatitis B surface antigen positive mothers and their babies: HBeAg passage through the placenta and the rate of decay in babies. J Med Virol. 2003;71:360-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 133] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Söderström A, Norkrans G, Lindh M. Hepatitis B virus DNA during pregnancy and post partum: aspects on vertical transmission. Scand J Infect Dis. 2003;35:814-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 94] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Xia GL, Gong J, Wang JJ, Meng ZD, Jia ZY, Cao HL, Liu CB. [Efficacy of recombinant hepatitis B vaccine and low-dose hepatitis B immune globulin in preventing mother-infant transmission of hepatitis B virus infection]. Zhonghua Liu Xing Bing Xue Za Zhi. 2003;24:362-365. [PubMed] |
| 7. | Chen HY, Shen DX, Wang SH, Wang XH. Detection on presence of hepatitis B virus in human infected by vertical transmission. Zhongguo Gonggong Weisheng Zazhi. 2002;18:417-419. |
| 8. | Wang XP, Xu DZ, Li YG, Yan YP, Men K, Zhang JX. HBsAg uptake into human trophoblasts cultured in vitro. DiYi JunYi DaXue XueBao. 2003;23:16-20. [PubMed] |
| 9. | Yu MM, Zhang Y, Wu GH, Huang CA, She JJ, Zhang JJ, Jiang KJ. Experimental studies on transmission of EHF in rodent. Zhonghua Yufang Yixue Zazhi. 1994;28:132-134. |
| 10. | Givens MD, Galik PK, Riddell KP, Brock KV, Stringfellow DA. Replication and persistence of different strains of bovine viral diarrhea virus in an in vitro embryo production system. Theriogenology. 2000;54:1093-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Tagawa M, Robinson WS, Marion PL. Duck hepatitis B virus replicates in the yolk sac of developing embryos. J Virol. 1987;61:2273-2279. [PubMed] |
| 12. | Zhao YW, Xu JZ, Yang ZG, Lang ZW, Yan HP, Huang ZH. Preparation of digoxignin labelled probe and detection of trans-fusion transmitted virus in extrahepatic tissue with in situ hybridization. Jiangsu Yixue Zazhi. 2002;26:428-430. |
| 13. | Zhou Sl, Zhao LS, Li H, Liu L. Detection of HBVDNA in oocyte of a patient with chronic hepatitis B by in situ by hybridization. J Heredity Disease. 1989;6:46-48. |
| 14. | Taylor PJ, Gill MJ, Mahadevan M, Pattinson HA. Hepatitis B virus in human follicular fluid. Fertil Steril. 1987;48:514. [PubMed] |
| 15. | Yamada M, Nakamura K, Nakajima Y, Yamamoto M, Komae H, Okuda K, Tsuji M, Arai M. Ground-glass hepatocytes in fibrinogen storage disease in Japanese Black calves. J Comp Pathol. 2002;126:95-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Alonso-Marti C, Moreno A, Barat A, Solera JC, Oliva H. Co-existence of hepatocyte ground-glass inclusions from several causes. Histopathology. 1990;16:304-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |