Published online Sep 28, 2005. doi: 10.3748/wjg.v11.i36.5714
Revised: December 15, 2004
Accepted: December 21, 2004
Published online: September 28, 2005
AIM: To identify the decreasing effect of xenotransplantion in combination with privileged sites on rejection and death of biological semipermeable membrane-(BSM) encapsulated implanted islets.
METHODS: After the BSM experiment in vitro, BSM-encapsulated SD rat’s islet-like cell clusters (ICCs) were xenotransplanted into normal dog’s brain. Morphological changes were observed under light and transmission electron microscope. The islets and apoptosis of implanted B cells were identified by insulin-TUNEL double staining.
RESULTS: The BSM used in our study had a favorable permeability, some degree of rigidity, lighter foreign body reaction and toxicity. The grafts consisted of epithelioid cells and loose connective tissue. Severe infiltration of inflammatory cells was not observed. The implanted ICCs were identified 2 mo later and showed typical apoptosis.
CONCLUSION: BSM xenotransplantation in combination with the privileged site can inhibit the rejection of implanted heterogeneous ICCs, and death of implanted heterogeneous B cells is associated with apoptosis.
- Citation: Xin ZL, Ge SL, Wu XK, Jia YJ, Hu HT. Intracerebral xenotransplantation of semipermeable membrane- encapsuled pancreatic islets. World J Gastroenterol 2005; 11(36): 5714-5717
- URL: https://www.wjgnet.com/1007-9327/full/v11/i36/5714.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i36.5714
Studies have shown that porcine ICCs xenotransplanted into rat’s brain could survive for only eighteen days[1,2], suggesting that the immuno-privileged function of brain cannot ensure the long-time survival of heterogeneous islets. Immunity-isolation is an effective way to lessen the immunological rejection damage[3,4]. Better effect can be achieved through the combination of different immunity-isolation techniques[5]. Rat’s pancreatic islet is easy to be separated, transgenic rats are also developed, and the cell line secreting insulin has been established[6]. However, the exact effects of apoptosis on the death of xenotransplanted islets cannot be shown[7-9]. In this study, BSM-encapsuled SD rat’s ICCs were xenotransplanted into a normal dog’s brain to identify their decreasing effect on rejection and death of implanted islets.
SD rats, weighing 50-200 g were used in this study. Pancreas and its amiculas and vessels were washed twice in Hank’s solution and then minced. The fragments were digested with collagenase V (1 g/L, Sigma) at 37°C for 15- 20 min, ground and filtrated through an 80-mesh steal net to remove connective tissue, washed in cold Hank’s solution to stop the activity of collagenase. Then the filtrates were filtrated with a 400-mesh steal net to remove the small exocrine cells, red and white blood cells with the bigger ICCs kept on the net. The cell clusters on the net were collected and put into the Ficoll fluid (Euro-Collin) at the concentrations of 25 g/L, 23 g/L, 20 g/L and 11 g/L. The mixed liquors were centrifuged at 3 500 r/min at 4°C for 15 min. ICCs were washed three times. The purity of ICCs obtained in this way was (88.2 ± 7.6) %. These ICCs were cultured in non-insulin DMEM supplemented with 10% fetal bovine serum, 2 mmol/L glutamine, 10 mmol/L nicotinamide, 5 mmol/L hydroxyethyl methylmethane sulfonic acid, 100 000 U/L penicillin and 1 g/L streptomycin, and maintained at 37°C in a high-humidity incubator with 50 mL/L CO2 . A total of 400-1 000 purified ICCs were obtained from each rat. These ICCs were stained with trypan blue and the activity was 90%.
Five semipermeable membrane encysts were filled with 1 mL of fresh blood plasma, put into 200 mL of blood and maintained at 37°C in a high-humidity incubator with 50 mL/L CO2 and kept agitated with magnetic force. Fluid in encysts was suctioned respectively 5 h later, then smeared and observed under a light microscope by Wright’s staining.
Eight semipermeable membrane encysts were filled with 1 mL of isotonic sodium chloride, put into 400 mL of isotonic sodium chloride containing 29.1 mmol/L glucose and kept agitated with magnetic force. The encysts were taken out at 1, 4, 8, 12, 16, 20, 24 and 28 min, respectively, and washed with isotonic sodium chloride. The fluid in the encysts was obtained and the concentration of glucose was detected.
About twelve thousands separated ICCs were divided into ten parts, five were encapsulated by BSM, five were routinely cultured. The culture medium was changed every three days. The concentration of insulin in the medium was detected on d 3, 6, and 15 respectively when the medium was changed.
After ICCs were cultured for 72 h, they were encapsulated by aseptic BSM. The volume of encysts was 1.5 cm × 1.5 cm × 1.5 cm. Each of them contained 6 000 to 10 000 ICCs.
The recipients were normal male local hybrid dogs weighing 7-14 kg. The dogs were intravenously anesthetized and their hair was shaved. The straight nick was made at frontal-occipital middle line perpendicular to the zygomatic arch. The temporalis muscles were pulled back to expose the skull, a hole was burred to enlarge the skull window to 3 cm × 3 cm, and the dura mater was cut open. The pallium was exposed to make a nick under the cerebral lateral fissure, and a fistula was made into ventricle between the rectal fissure and rectal lateral fissure to form a 2 cm × 2 cm × 1.5 cm tunnel. The brain tissue was wet compressed with 0.5 g/L orthophosphoric acid dexamethasone for 15 min[7] , then encysts were transplanted into the tunnel. The dura mater was relocated and compressed with gelatin sponges from the outside. Scalp and muscles were then sutured. Dogs were allowed to take food when consciousness was regained after anesthesia. Penicillin and streptomycin were used 30 min before and 1-3 d after operation.
The dogs were divided into control group ( n = 2) and experimental group( n = 4). In control group, a fistula was made, the stoma at the ventricle side was covered with gelatin sponges. Then the encysts were cut open to let the contents efflux into the brain tissue and the stoma at cortex side was covered with gelatin sponges. In experimental group, brain tissue was obtained 1 mo and 2 mo after encapsulated ICCs were transplanted.
According to the schedule, the brain was perfused with 4 g/L paraformaldehyde phosphate buffer through the ambi-common carotid artery. The grafts’ shape, color, location and changes of surrounding brain tissue were observed. Then part of the grafts and surrounding brain tissue were paraffin embedded, sectioned and stained with HE. Morphological changes of grafts and brain tissue were observed under a light microscope. Some grafts were divided into 1 mm × 1 mm × 1 mm piecers. These pieces were fixed with 25 g/L glutaraldehyde, dehydrated, embedded and then cut into ultrathin sections. The ultramicro-changes of the grafts were observed under a transmission electron microscope (JEM2000).
The first antibody was replced by normal mouse serum in the negative control group. Paraffin-embedded sections were used. The first antibody was mouse anti-human insulin used at 1:100 dilution and stained according to the avidin-biotin compound method (ABC), and then counterstained with hematoxylin to identify B cells in the grafts. The DNA fragmentation of transplanted B cells was identified by insulin-TUNEL double staining .
The BSM was not damaged during the whole process of the experiment in vitro. In lymphocyte-diffusion experiment, lymphocytes and any other blood cells were not immersed into the semipermeable membrane capsule, but glucose could rapidly diffuse into the capsule. The concentration of glucose in the capsule was 14.3 mmol/L after 8 min, which was 50% of the concentration out of the capsule. After 28 min. The concentration of glucose in the capsule reached the same environmental level. The concentration of insulin on d 3, 6 and 15 was (139.0 ± 3.4) mU/L, (78.7 ± 3.9) mU/L and (66.2 ± 2.9) mU/L, respectively in the experimental group , and (145.4 ± 4.2) mU/L, (79.4 ± 2.1) mU/L and (48.2 ± 4.1) mU/L, respectively in the control group. There was no conspicuous variability (P > 0.05) between the two groups at various time points. Insulin secreted from pancreatic islet cells could diffuse rapidly out of the capsule.
The dogs of both groups had no conspicuous behavior disorder. No dog in the experimental group was infected. The graft was deep gray and homogenous in the experimental group. It was connected with the ventricle at the inner side and adhered to the thickening dura mater at the lateral side as well as in contact with the cerebral parenchyma. The boundary was clear. Brain tissue around the graft had no obvious hemorrhage, necrosis and inflammatory abscess. Only residual semipermeable membrane was observed in the control dogs.
Under light microscope, the graft was consisted of epithelial cells and loose connective tissue, including epithelial cells, fibroblasts, collagen fiber, and small vessels. In the middle of them, there were lymphocytes and other kinds of white blood cells. Epithelial cells mostly adhered to the fibroblasts, collagen fiber or small vessels, and were arranged loosely in the connective tissue along the collagen fibers. The structure of small vessels was normal, while that of some parts of the semipermeable membrane was different, but there was no clump-like infiltration of lymphocytes.
Slight hyperplasia and hydropsia of glial cells were observed in the brain tissue around the graft, but severe infiltration of inflammatory cells was not observed.
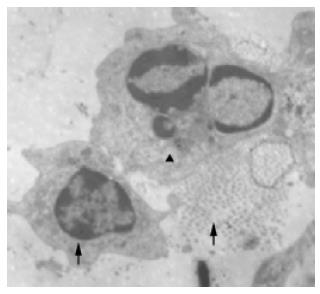
Under electron microscope, secreting granulae were observed in the endochylema. The granulae were not only different in size but also diferent from those of pancreatic islet cells. Intact and contracted caryons, aggregated chromosome and typical apoptotic bodies were found in some cells (Figure 1).
No xenotransplanted cell disappeared in the control group.
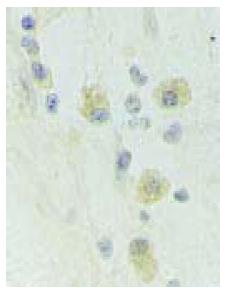
Cytoplasms of many epithelial cells in the grafts of EG were stained yellow, indicating that they were islet B cells (Figure 2). The implanted B cells showed typical apoptosis by insulin-TUNEL double staining.
Studies have shown that porcine ICCs xenotransplanted into a rat’s brain can survive for only 18 d[1,2], and fetal porcine ICCs transplanted into cynomolgus monkey’s kidney encysts are completely rejected on d 6[5,8]. In the present study, no infiltration of clump-like polymorphic nuclear cells and lymphocytes was found, suggesting that rejection occurs in xenotransplanted ICCs. Immunohistochemical staining showed that pancreatic islet B cells still existed in the grafts after 2 mo. The dogs had no conspicuous behavior disorder after transplantation. Slight hyperplasia and hydropsia of glial cells were observed in the brain tissue around the graft.
These facts suggest that ing Xenotransplantation of BSM in combination with the privileged site can inhibit the rejection of implanted heterogeneous ICCs.
In the present study, cells displayed different morphological features in different environments to adapt themselves to the microenvironment. The culture medium was gradually absorbed after graffing and replaced by loose connective tissue until solidification. The appearance of connective tissue was a kind of compensatory reaction, giving support and nutrition to islet cells and keeping them stable. Islet cells were arranged loosely in the connective tissue along the collagen fibers, while the in situ compact cell clumps were observed under the pressure of exocrine division.
It is easier to transplant pancreatic islets when they are capsuled and to dislodge them when the rejection is serious during the early period of transplantation. This is very important when a clinical trial is carried out. According to the in vitro experiments and transplant experiments, the semi permeable membrane used in our study had a favorable permeability, some degree of rigidity, lighter foreign body reaction and toxicity.
It was reported that cerebrospinal fluid (CSF) could ensure islet survival with sufficient oxygen and nutrients[10]. We made a tunnel into the ventricle, which could connect capsules with CSF immediately. Accordingly, islets were supplied with nutrients in the earlier period, which is beneficial for their survival. However, how many islet nutrients can be obtained in this way remians unclear. We also found that the implanted B cells showed classic apoptosis by insulin-TUNEL double staining and electron microscopy. The death of implanted heterogeneous B cells is associated with apoptosis. Why apoptosis occurs and how to delay or block this apoptosis are the subjects to be studied[11-13].
Surgical procedures injure brain tissue and integrality of the blood-brain barrier. Wet compressing with dexamethasone on the injured brain can decrease the blood-brain barrier permeability and non-specific exudation of inflammatory cells[14].
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Triponez F, Oberholzer J, Morel P, Toso C, Yu D, Cretin N, Buhler L, Majno P, Mentha G, Lou J. Xenogeneic islet re-transplantation in mice triggers an accelerated, species-specific rejection. Immunology. 2000;101:548-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Thomas FT, Hutchings A, Contreras J, Wu J, Jiang XL, Eckhoff D, Thomas JM. Islet transplantation in the twenty-first century. Immunol Res. 2002;26:289-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Opara EC, Kendall WF. Immunoisolation techniques for islet cell transplantation. Expert Opin Biol Ther. 2002;2:503-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Bucher P, Mathe Z, Bosco D, Andres A, Bühler LH, Morel P, Berney T. Islet of Langerhans transplantation for the treatment of type 1 diabetes. Swiss Surg. 2003;9:242-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Juang JH. Islet transplantation: an update. Chang Gung Med J. 2004;27:1-15. [PubMed] |
| 6. | Xin ZL, Jia YJ, Hu HT, Ren HM. The coculture of pancreatic islets with neurons and gliacytes. Xi'an Yike Daxue Xuebao. 2001;22:475-479. |
| 7. | Lehnert AM, Yi S, Burgess JS, O'Connell PJ. Pancreatic islet xenograft tolerance after short-term costimulation blockade is associated with increased CD4+ T cell apoptosis but not immune deviation. Transplantation. 2000;69:1176-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Vizzardelli C, Molano RD, Pileggi A, Berney T, Cattan P, Fenjves ES, Peel A, Fraker C, Ricordi C, Inverardi L. Neonatal porcine pancreatic cell clusters as a potential source for transplantation in humans: characterization of proliferation, apoptosis, xenoantigen expression and gene delivery with recombinant AAV. Xenotransplantation. 2002;9:14-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Xin ZL, Jia YJ, Hu HT, Ren HM. Rat ICCs apoptosis of xenotransplantation into dogs brain. Disi Junyi Daxue Xuebao. 2002;23:2279-2281. |
| 10. | Atwater I, Yañez A, Cea R, Navia A, Jeffs S, Arraya V, Szpak-Glasman M, Leighton X, Goping G, Bevilacqua JA. Cerebral spinal fluid shunt is an immunologically privileged site for transplantation of xenogeneic islets. Transplant Proc. 1997;29:2111-2115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Saudek F. Gene therapy in the treatment of diabetes mellitus. Cas Lek Cesk. 2003;142:523-527. [PubMed] |
| 12. | Contreras JL, Bilbao G, Smyth C, Eckhoff DE, Xiang XL, Jenkins S, Cartner S, Curiel DT, Thomas FT, Thomas JM. Gene transfer of the Bcl-2 gene confers cytoprotection to isolated adult porcine pancreatic islets exposed to xenoreactive antibodies and complement. Surgery. 2001;130:166-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 70] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Brandhorst D, Hammes HP, Brandhorst H, Zwolinski A, Nahidi F, Alt A, Bretzel RG. Pretransplant induction of HSP-70 in isolated adult pig islets decreases early islet xenograft survival. Cell Transplant. 2000;9:423-430. [PubMed] |
| 14. | Xin ZL, Duan GR, Lv J, Yang QY. Experimental study of local use of dexamethasone and its effects on brain edema and blood brain barrier permeability following brain injury. Zhonghua Shiyan Waike Zazhi. 1997;14:355-356. |