Published online Sep 28, 2005. doi: 10.3748/wjg.v11.i36.5696
Revised: January 23, 2005
Accepted: January 26, 2005
Published online: September 28, 2005
AIM: Esophageal squamous cell carcinoma is generally sensitive to chemoradiotherapy (CRT), but some cases are not. Using a retrospective analysis, we aimed to identify the predictors of the response by esophageal squamous cell carcinoma to definitive CRT.
METHODS: The intensities of expression of p53, Ki67, Bcl-2, Bax, cyclin D1, VEGF, CDC25B, and metallothionein (MT) were evaluated immunohistochemically in the biopsy specimens obtained before CRT, and the intensities of their expression were tested for correlations with the clinical effects of CRT.
RESULTS: The esophageal squamous cell carcinomas with negative p53, positive CDC25B, and negative MT expression were found to be significantly more sensitive to CRT. In addition, p53 positivity and CDC25B positivity respond well to CRT.
CONCLUSION: Esophageal squamous cell carcinomas with negative p53,positive CDC25B, and negative MT expressions respond well to CRT. Even with p53 positivity, if with CDC25B positivity, CRT can be expected.
- Citation: Sunada F, Itabashi M, Ohkura H, Okumura T. p53 negativity, CDC25B positivity, and metallothionein negativity are predictors of a response of esophageal squamous cell carcinoma to chemoradiotherapy. World J Gastroenterol 2005; 11(36): 5696-5700
- URL: https://www.wjgnet.com/1007-9327/full/v11/i36/5696.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i36.5696
Chemoradiotherapy (CRT) is one of the most commonly used modalities of treatment for squamous cell carcinoma of the esophagus. Although the response rate to CRT with 5-fluorouracil and cisplatin is high (64%), there is no survival benefit for non-responders[1]. Hence, it would be useful to be able to predict the response to CRT so that the non-responders could avoid the side-effects of CRT. This has not been possible, however, on the basis of the clinicopathological factors that are routinely examined. Recent advances in tumor biology, especially in relation to apoptosis, offer a possible solution to this problem. Apoptosis induced by CRT involves various biological phenomena, such as DNA repair, altered drug metabolism, inflammation, molecular chaperoning and changes to the cell cycle, and a variety of biological markers, including p53, Ki67, Bcl-2, Bax, VEGF, cyclin D1, metallo-thionein (MT), and CDC25B have been investigated for an association with response to CRT[2-5]. p53 is a tumor suppressor gene that contributes to the preservation of genetic stability by facilitating both G1 arrest and apoptosis in response to DNA damage[6,7]. The Bcl-2 family of proteins includes molecules with both the anti-apoptotic effects (e.g., Bcl-2 and Bcl-XL) and pro-apoptotic effects (e.g., Bax and Bak), and cell susceptibility to apoptosis has been found to be determined by competing dimerization of different members of the Bcl-2 family[8,9]. Previous studies have revealed that CDC25B, which activates CDC2 and the G2-M progression, is significantly associated with radiation sensitivity among various molecules regulating the G2-M checkpoint[10]. MT is an intracellular metal-binding protein involved in zinc homeostasis and the detoxification of heavy metals[11]. A study has shown that MT affects cisplatin-induced apoptosis[12]. Moreover, another recent study has reported that essential cytotoxic targets of both oxidants and heavy metals exist in the cytoplasm and establish the importance of nucleocytoplasmic partitioning for the function of MT[13], and a positive association has also been found between MT expression and resistance to CRT[14]. Thus, the purpose of our study was to identify the predictors of the response to definitive CRT with a retrospective analysis. Therefore, we have investigated a variety of biological markers, including p53, Ki67, Bcl-2, Bax, VEGF, cyclin D1, MT, and CDC25B.
Thirty-six patients with advanced squamous cell carcinoma of the esophagus, who refused surgery and gave informed consent to CRT at Ibaraki Prefectural Central between 1996 and 2001, were included in this study (Table 1). The diagnosis of squamous cell carcinoma was confirmed by histological examination of biopsy specimens obtained before starting CRT (the clinicopathological data are summarized in Table 1). Response to CRT was evaluated clinically after two courses and the evaluation included a barium esophagogram, esophagoscopy, and computed tomography (CT) of the chest and abdomen. A complete response was defined as no visible tumor by esophagoscopy, biopsy specimens free of tumor tissue, and normal CT findings; a partial response as >50% tumor regression as evaluated by CT, and >50% reduction of intraesophageal tumor extension assessed by barium swallow and esophagoscopy; no change as < 50% regression of tumor extension, and no evidence of tumor progression; and progressive disease as increasing tumor growth indicated by barium swallow or esophagoscopy and increasing tumor diameter assessed by CT.
| Parameters | Values |
| Sex (male/female) | Mar-33 |
| Age (range) | 63.3±9.2 (42-78 yr) |
| Histopathology | |
| Well-differentiated | 6 |
| Moderately differentiated | 23 |
| Poorly differentiated | 7 |
| Stage (UICC) | |
| I | 4 |
| II | 7 |
| III | 18 |
| IVa | 7 |
Chemotherapy consisted of protracted infusion of 5-FU at a dose of 400 mg/m2 per d on d 1-5 and 8-12, combined with a 2-h infusion of CDDP at 40 mg/m2 per d on d 1 and 8, repeated twice every 5 wk. Concurrent radiotherapy was started on d 1 at 2 Gy/d for 5 d/wk. The total radiation dose was 60 Gy, with a 2-wk break after a dose of 30 Gy. The patients were followed up every 3 mo for the first 3 years after the end of treatment, and afterward every 6 mo thereafter. New chemotherapy agent (e.g., docetaxel) was applied for the patient with non-effective CRT.
Immunostainings for p53, Ki67, Bcl-2, Bax, cyclin D1, VEGF, MT, and CDC25B were performed using streptavidin-peroxidase complex methods with an EnVision+™ peroxidase kit (Dako, Glostrup, Denmark) on a TeckMate Horizon automated staining system (Dako, Glostrup, Denmark). Primary antibodies were incubated overnight at 4°C with E9 (dilution 1:50; Dako, Kyoto, Japan) for MT, and for 1 h at room temperature with DO-7 (dilution 1:50; Novocastra Laboratories, Newcastle upon Tyne, UK) for p53, with Ki-S5 (dilution 1:50; Dako, Kyoto, Japan) for Ki- 67, with 124 (dilution 1:50; Dako, Kyoto, Japan) for Bcl-2, with A3533 (dilution 1:50; Dako, Kyoto, Japan) for Bax, with DSC-6 (dilution 1:200; Novocastra) for cyclin D1, with JH 121 (dilution 1:50; Upstate, Lake Placid, NY, USA) for VEGF, and with C45820 (5 µ g/mL; Transduction Laboratories, Lexington, KY, USA) for CDC25B. The expressions of p53, Bcl-2, Bax, VEGF, cyclin D1, MT, and CDC25B were investigated in consecutive histological sections prepared from the biopsy specimens. After being pretreated thrice, each for 5 min, in a citrate buffer (pH 6.0) at 750 W, the slides were separately incubated overnight at 4°C with the mAbs, and the expressions of p53, Ki-67, Bcl-2, Bax, VEGF, cyclin D1, MT, and CDC25B were assessed under light microscope by one observer (Itabashi), who was unknown about the clinical outcome. The percentages of positive tumor cells were determined semiquantitatively, and each sample was assigned to one of the following categories: negative (0-10%) and positive (11-100%). In addition, the intensities of immunostaining of antigens localized in the cytoplasm (Bcl-2, Bax, cyclin D1, VEGF, MT, and CDC25B) were classified as negative or positive. Staining intensity was not determined for p53 (nuclear immunostaining), because no significant differences in staining intensity were observed in the p53-positive cases.
Associations between two parameters were analyzed by the Spearman’s rank correlation test. For continuous parameters, the difference between the two groups was analyzed by Student’s t-test. Determination of the distribution of immunohistoc-hemical staining between groups was analyzed using χ2 analysis. Cumulative survival of the patients was calculated on July 31, 2002, by the Kaplan-Meier method and the statistical significance was analyzed by the log-rank test. P<0.05 was considered statistically significant in all analyses. All statistical analyses were performed using the StatView version 5.0 software package (Abacus Concepts, Berkeley, CA, USA).
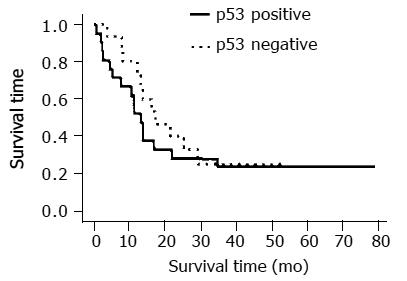
The overall proportions of the cells positive for expression of p53, Bcl-2, Bax, Ki67, cyclin D1, VEGF, MT, and CDC25B were 58.3%, 27.8%, 30.6%, 86.1%, 41.7%, 58.3%, 27.8%, and 13.9%, respectively (Tables 2 and 3). Not MT negativity, but CDC25B positivity with p53 positivity is a predictor of a response to CRT ( respectively p=0.39, 0.027).The median survival time of the p53-negative and p53-positive patients were 588 and 415 d, respectively (P<0.001, Student’s t-test), but no significant difference was found between the survival curves of the p53-negative and p53-positive patients and esophageal cancer staging (Figures 1 and 2). However, there was a significant association between p53-negative and MT-negative patients and effect of CRT (Table 4) . At the end of the follow-up period on July 31, 2002, 25% patients (9/36) were still alive. The follow-up time for all 36 patients ranged from 26.7 to 81 mo (median, 59.5 ± 18.1 mo). In the group treated by CRT, there was a marked difference in mean survival time between the patients with p53- negative tumors (19.6 ± 10.3 mo) and with p53-postive tumors (13.8 ± 8. 6 mo). Esophageal squamous cell carcinomas relapsed in 11 out of 25 (44%) responder cases after CRT.
| Markers | Responder | Non-responder | P |
| (CR+PR) | (NC+PD) | ||
| p53(-) | 14 | 1 | 0.0095 |
| p53(+) | 11 | 10 | |
| MT (-) | 21 | 5 | 0.019 |
| MT (+) | 4 | 6 | |
| CDC25B (-) | 7 | 8 | 0.0129 |
| CDC25B (+) | 18 | 3 |
| Markers | Responder | Non-responder | P |
| (CR+PR) | (NC+PD) | ||
| Bax (-) | 17 | 8 | 0.799 |
| Bax (+) | 8 | 3 | 0.799 |
| Bcl-2 (-) | 18 | 8 | 0.836 |
| Bcl-2 (+) | 7 | 3 | 0.836 |
| Cyclin D1 (-) | 15 | 6 | 0.305 |
| Cyclin D1 (+) | 10 | 5 | 0.305 |
| VEGF (-) | 10 | 5 | 0.936 |
| VEGF (+) | 15 | 6 | 0.936 |
| Ki67 (-) | 4 | 1 | 0.586 |
| Ki67 (+) | 21 | 10 | 0.586 |
| Immunoreactivity/response | P | ||||
| p53 | - | - | + | + | 0.027 |
| CDC25B | + | - | + | - | |
| Responder | 9 | 5 | 9 | 2 | |
| Non-responder | 0 | 1 | 3 | 7 | |
| MT | - | - | + | + | |
| 0.0305 | |||||
| CDC25B | + | - | + | - | |
| Responder | 16 | 5 | 2 | 2 | |
| Non-responder | 2 | 3 | 1 | 5 | |
Esophageal cancer is a relatively uncommon but aggressive disease. Surgical resection has been widely accepted as the standard treatment for esophageal cancer, and techniques have improved during the past decades. However, long-term survival after resection of carcinoma of the thoracic esophagus is generally poor, with rates of only 20-42.4%[15-18]. Some reports on CRT have indicated that it offers various advantages in managing esophageal cancer[19]. Oncologists have advocated a non-surgical approach with definitive CRT as the standard treatment for this disease[20-23]. The value of CRT for the treatment of unresectable esophageal squamous cell carcinoma remains a matter of controversy. Only a few clinical studies have been published since the 1980s, and most patients in those studies had local-regional disease (UICC stage I or II). Several investigators have reported successful results with these modalities, either with or without surgery, against local-regional carcinoma[24-26]. The combination of 5-FU and CDDP has become the standard regimen, not only because of the clinical outcome but also because of the synergism between the two agents and their radiosensitizing effects[27,28]. Recently published results on CRT have indicated that it offers various advantages for the treatment of carcinoma of the esophagus[29,30]. A multicenter study on the indications for CRT as curative therapy for patients with locally advanced disease has suggested that concurrent CRT is potentially curative, even in cases of locally advanced carcinoma of the esophagus (i.e., T4 and/or M1 lymph node metastasis disease)[31]. Some studies have implicated various molecules, including p53, CDC25B, and MT, as candidates for biological markers for the response of human esophageal cancer to CRT[10,14]. We evaluated the role of cell-cycle-regulating molecules in the sensitivity of human squamous cell carcinoma of the esophagus to CRT by immunohistochemical methods and found p53, MT, and CDC25B to be significant independent markers for predicting sensitivity to CRT. Our results showed that negative immunostaining for p53 in pre-CRT biopsy specimens predicted a good response. In addition to being the only established biological marker for response to CRT in clinical studies, p53 is the best characterized and most powerful marker[32,33], and it is commonly acknowledged to be a definite indicator of radiation sensitivity in various cancers[34,35]. The p53 immunoreactivity is generally thought to be attributable to the accumulation of abnormal p53 protein[36]. In our results, even the tumors with p53 positivity were sensitive to the CRT. In most of the esophageal squamous cell carcinoma, p53 are positive immunohistologically. Even with p53 positivity, CDC25B positivity respond well to CRT. So, we think that another pathway independent of p53 that regulate CRT sensitivity is sure to exist. G2-M checkpoint, in which CDC25B is involved, is a candidate for this p53-independent pathway. Among the various molecules that regulate G2-M arrest, overexpression of CDC25B has frequently been observed in human cancer[37,38], and also found to be related with response to CRT[10]. In our cases, no significant difference was observed between the survival curves of the p53 -negative and p53-positive patients. It might be because a new chemotherapeutic agent (e.g., docetaxel) was applied for the patients with non-effective CRT. Docetaxel is an active drug for treating squamous cell carcinoma of head and neck. For the patients with recurrent or metastatic disease of squamous cell carcinoma, docetaxel could be used as a second line chemotherapy[39]. From a clinical standpoint, it is very important that a prospective study based on this result should be done. There are only retrospective studies that have been reported up to now. Standard therapy may be developed from the results of a prospective study based on the immunohistochemical characteristics of the tumor biopsy.
Science Editor Kumar M and Guo SY Language Editor Elsevier HK
| 1. | Yano M, Tsujinaka T, Shiozaki H, Inoue M, Doki Y, Yamamoto M, Tanaka E, Inoue T, Monden M. Concurrent chemotherapy (5-fluorouracil and cisplatin) and radiation therapy followed by surgery for T4 squamous cell carcinoma of the esophagus. J Surg Oncol. 1999;70:25-32. [PubMed] |
| 2. | Sarbia M, Stahl M, Fink U, Willers R, Seeber S, Gabbert HE. Expression of apoptosis-regulating proteins and outcome of esophageal cancer patients treated by combined therapy modalities. Clin Cancer Res. 1998;4:2991-2997. [PubMed] |
| 3. | Kitadai Y, Amioka T, Haruma K, Tanaka S, Yoshihara M, Sumii K, Matsutani N, Yasui W, Chayama K. Clinicopathological significance of vascular endothelial growth factor (VEGF)-C in human esophageal squamous cell carcinomas. Int J Cancer. 2001;93:662-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 152] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 4. | Rosa AR, Schirmer CC, Gurski RR, Meurer L, Edelweiss MI, Kruel CD. Prognostic value of p53 protein expression and vascular endothelial growth factor expression in resected squamous cell carcinoma of the esophagus. Dis Esophagus. 2003;16:112-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Güner D, Sturm I, Hemmati P, Hermann S, Hauptmann S, Wurm R, Budach V, Dörken B, Lorenz M, Daniel PT. Multigene analysis of Rb pathway and apoptosis control in esophageal squamous cell carcinoma identifies patients with good prognosis. Int J Cancer. 2003;103:445-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6088] [Cited by in RCA: 6304] [Article Influence: 197.0] [Reference Citation Analysis (0)] |
| 7. | Caelles C, Helmberg A, Karin M. p53-dependent apoptosis in the absence of transcriptional activation of p53-target genes. Nature. 1994;370:220-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 603] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 8. | Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899-1911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2769] [Cited by in RCA: 2759] [Article Influence: 106.1] [Reference Citation Analysis (0)] |
| 10. | Miyata H, Doki Y, Shiozaki H, Inoue M, Yano M, Fujiwara Y, Yamamoto H, Nishioka K, Kishi K, Monden M. CDC25B and p53 are independently implicated in radiation sensitivity for human esophageal cancers. Clin Cancer Res. 2000;6:4859-4865. [PubMed] |
| 11. | Hamer DH. Metallothionein. Annu Rev Biochem. 1986;55:913-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1379] [Cited by in RCA: 1239] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 12. | Kondo Y, Rusnak JM, Hoyt DG, Settineri CE, Pitt BR, Lazo JS. Enhanced apoptosis in metallothionein null cells. Mol Pharmacol. 1997;52:195-201. [PubMed] |
| 13. | Woo ES, Lazo JS. Nucleocytoplasmic functionality of metallothionein. Cancer Res. 1997;57:4236-4241. [PubMed] |
| 14. | Yamamoto M, Tsujinaka T, Shiozaki H, Doki Y, Tamura S, Inoue M, Hirao M, Monden M. Metallothionein expression correlates with the pathological response of patients with esophageal cancer undergoing preoperative chemoradiation therapy. Oncology. 1999;56:332-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Ando N, Ozawa S, Kitagawa Y, Shinozawa Y, Kitajima M. Improvement in the results of surgical treatment of advanced squamous esophageal carcinoma during 15 consecutive years. Ann Surg. 2000;232:225-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 408] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 16. | Müller JM, Erasmi H, Stelzner M, Zieren U, Pichlmaier H. Surgical therapy of oesophageal carcinoma. Br J Surg. 1990;77:845-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 662] [Cited by in RCA: 624] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 17. | Akiyama H, Tsurumaru M, Udagawa H, Kajiyama Y. Radical lymph node dissection for cancer of the thoracic esophagus. Ann Surg. 1994;220:364-72; discussion 372-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 634] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 18. | Turnbull AD, Ginsberg RJ. Options in the surgical treatment of esophageal carcinoma. Chest Surg Clin N Am. 1994;4:315-329. [PubMed] |
| 19. | Coia LR. Chemoradiation as primary management of esophageal cancer. Semin Oncol. 1994;21:483-492. [PubMed] |
| 20. | Herskovic A, Martz K, al-Sarraf M, Leichman L, Brindle J, Vaitkevicius V, Cooper J, Byhardt R, Davis L, Emami B. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med. 1992;326:1593-1598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1514] [Cited by in RCA: 1444] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 21. | Cooper JS, Guo MD, Herskovic A, Macdonald JS, Martenson JA, Al-Sarraf M, Byhardt R, Russell AH, Beitler JJ, Spencer S. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA. 1999;281:1623-1627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1261] [Cited by in RCA: 1368] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 22. | Araújo CM, Souhami L, Gil RA, Carvalho R, Garcia JA, Froimtchuk MJ, Pinto LH, Canary PC. A randomized trial comparing radiation therapy versus concomitant radiation therapy and chemotherapy in carcinoma of the thoracic esophagus. Cancer. 1991;67:2258-2261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Wilson KS, Lim JT. Primary chemo-radiotherapy and selective oesophagectomy for oesophageal cancer: goal of cure with organ preservation. Radiother Oncol. 2000;54:129-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Leichman L, Herskovic A, Leichman CG, Lattin PB, Steiger Z, Tapazoglou E, Rosenberg JC, Arbulu A, Asfaw I, Kinzie J. Nonoperative therapy for squamous-cell cancer of the esophagus. J Clin Oncol. 1987;5:365-370. [PubMed] |
| 25. | Leichman L, Steiger Z, Seydel HG, Dindogru A, Kinzie J, Toben S, MacKenzie G, Shell J. Preoperative chemotherapy and radiation therapy for patients with cancer of the esophagus: a potentially curative approach. J Clin Oncol. 1984;2:75-79. [PubMed] |
| 26. | Poplin E, Fleming T, Leichman L, Seydel HG, Steiger Z, Taylor S, Vance R, Stuckey WJ, Rivkin SE. Combined therapies for squamous-cell carcinoma of the esophagus, a Southwest Oncology Group Study (SWOG-8037). J Clin Oncol. 1987;5:622-628. [PubMed] |
| 27. | Scanlon KJ, Newman EM, Lu Y, Priest DG. Biochemical basis for cisplatin and 5-fluorouracil synergism in human ovarian carcinoma cells. Proc Natl Acad Sci USA. 1986;83:8923-8925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 220] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 28. | Byfield JE. Combined modality infusional chemotherapy with radiation. in: cancer chemotherapy by infusion, Lokich JJ (ed) 2nd edn. Chicago, IL: Percepta Press 1990; 521-551. |
| 29. | Forastiere AA, Orringer MB, Perez-Tamayo C, Urba SG, Zahurak M. Preoperative chemoradiation followed by transhiatal esophagectomy for carcinoma of the esophagus: final report. J Clin Oncol. 1993;11:1118-1123. [PubMed] |
| 30. | Coia LR. Chemoradiation as primary management of esophageal cancer. Semin Oncol. 1994;21:483-492. [PubMed] |
| 31. | Ohtsu A, Boku N, Muro K, Chin K, Muto M, Yoshida S, Satake M, Ishikura S, Ogino T, Miyata Y. Definitive chemoradiotherapy for T4 and/or M1 lymph node squamous cell carcinoma of the esophagus. J Clin Oncol. 1999;17:2915-2921. [PubMed] |
| 32. | Perdomo JA, Naomoto Y, Haisa M, Fujiwara T, Hamada M, Yasuoka Y, Tanaka N. In vivo influence of p53 status on proliferation and chemoradiosensitivity in non-small-cell lung cancer. J Cancer Res Clin Oncol. 1998;124:10-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Shimoyama S, Konishi T, Kawahara M, Aoki F, Harada N, Shimizu S, Murakami T, Kaminishi M. Expression and alteration of p53 and p21(waf1/cip1) influence the sensitivity of chemoradiation therapy for esophageal cancer. Hepatogastroenterology. 1998;45:1497-1504. [PubMed] |
| 34. | Hamada M, Fujiwara T, Hizuta A, Gochi A, Naomoto Y, Takakura N, Takahashi K, Roth JA, Tanaka N, Orita K. The p53 gene is a potent determinant of chemosensitivity and radiosensitivity in gastric and colorectal cancers. J Cancer Res Clin Oncol. 1996;122:360-365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 82] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Bergh J, Norberg T, Sjögren S, Lindgren A, Holmberg L. Complete sequencing of the p53 gene provides prognostic information in breast cancer patients, particularly in relation to adjuvant systemic therapy and radiotherapy. Nat Med. 1995;1:1029-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 283] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 36. | Begg AC, Hofland I, Moonen L, Bartelink H, Schraub S, Bontemps P, Le Fur R, Van Den Bogaert W, Caspers R, Van Glabbeke M. The predictive value of cell kinetic measurements in a European trial of accelerated fractionation in advanced head and neck tumors: an interim report. Int J Radiat Oncol Biol Phys. 1990;19:1449-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 137] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 37. | Galaktionov K, Lee AK, Eckstein J, Draetta G, Meckler J, Loda M, Beach D. CDC25 phosphatases as potential human oncogenes. Science. 1995;269:1575-1577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 399] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 38. | Gasparotto D, Maestro R, Piccinin S, Vukosavljevic T, Barzan L, Sulfaro S, Boiocchi M. Overexpression of CDC25A and CDC25B in head and neck cancers. Cancer Res. 1997;57:2366-2368. [PubMed] |
| 39. | Calais G. [Docetaxel and squamous cell carcinoma of the head and neck]. Bull Cancer. 2004;91:167-171. [PubMed] |