Published online Sep 28, 2005. doi: 10.3748/wjg.v11.i36.5614
Revised: December 20, 2004
Accepted: December 23, 2004
Published online: September 28, 2005
AIM: To investigate the anti-tumor effect of dendritic cells (DCs) pulsed with hsp70-peptide complexes derived from human hepatocellular carcinoma (HCC) cells on human T cells.
METHODS: Hsp70-peptide complexes were purified from human HCC cells with column chromatography using ADP-agarose and DEAE-Sepharose. DCs were derived from peripheral blood mononuclear cells of healthy donors in the presence of human GM-CSF and IL-4. The anti-tumor effect of DCs pulsed with hsp70-peptide complexes on human T-cell was assayed by CTL and enzyme-linked immunospot (ELISPOT) tests.
RESULTS: Hsp70-peptide complexes derived from human HCC cells activated phenotypic and functional maturation of DCs. The matured DCs stimulated a high level of autologous T-cell proliferation and type I cytokine secretion, and induced HCC-specific cytotoxic T lymphocytes (CTLs), which specifically killed HCC cells by a MHC class I restricted mechanism.
CONCLUSION: Hsp70-peptide complexes derived from human HCC cells can serve as a potent tumor antigen source for pulsing DCs, the pulsed DCs are very effective in activating specific T-cell responses against HCC cells.
- Citation: Wang XH, Qin Y, Hu MH, Xie Y. Dendritic cells pulsed with hsp70-peptide complexes derived from human hepatocellular carcinoma induce specific anti-tumor immune responses. World J Gastroenterol 2005; 11(36): 5614-5620
- URL: https://www.wjgnet.com/1007-9327/full/v11/i36/5614.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i36.5614
Hepatocellular carcinoma (HCC) is one of the most common malignancies in the world, especially in sub-Saharan Africa and Southeast Asia, and it rarely responds to conventional treatment, such as surgery, radiation, or chemotherapy[1,2]. The ability of tumors to escape from immune surveillance contributes to their uncontrolled growth, recurrence, and metastasis[3]. Finding an efficient therapeutic method for HCC has become the focus of researchers around the world. An ideal cancer therapy should have the potency to eradicate systemic tumors in multiple sites in the body and the specificity to discriminate between neoplastic and non-neoplastic cells. In these respects, immunotherapy that stimulates tumor-specific immune responses has become an attractive approach.
Dendritic cells (DCs) are professional antigen presenting cells (APCs), which direct the cellular immune response through antigen presentation in the presence of appropriate costimulation. These cells reside in an immature state in non-lymphoid tissues, where they can efficiently capture invading pathogens. After antigen uptake, they rapidly migrate to the draining secondary lymphoid tissues and convert into mature DCs with decreased antigen processing ability, enhanced expression of MHC and costimulatory molecules, and increased capacity of priming naïve T cells and initiating an adaptive immune response[4]. The extraordinary functional profile of DCs suggests that they may represent an ideal vector in the immunotherapy of cancer and infectious diseases[4]. Studies have shown that DC-based vaccines can induce potent anti-tumor immunity. Various strategies have been developed to introduce tumor-specific antigens into DCs and thereby to generate cytotoxic T lymphocyte (CTL) responses against malignant cells.
Heat shock proteins (HSPs) are important molecular chaperones that play essential roles in the regulation of protein synthesis and folding, and vesicular trafficking[5-7]. They are ubiquitously expressed at a basal level but are specifically induced in response to various stress conditions such as heat, anoxia, and metabolic stress[3]. The observation that preparations of HSPs from various tumors can elicit specific anti-tumor immune responses suggests their immunotherapeutic activity[8]. The observed immunogenicity of HSP preparations is derived from the antigenic peptides they chaperone[9]. Studies concerning the mechanisms of HSP preparations’ immunogenicity have shown that HSPs transfer antigenic peptides to professional APCs, such as DCs, and channel them into the class I antigen presenting pathway through receptors[10-14]. HSPs can also elicit cytokine production by, and adhesion molecule expression of, a range of cell types, and they can deliver maturation signals to APCs through receptor-mediated interactions[5,11,15].
In the current study, we used DCs pulsed with hsp70-peptide complexes derived from human HCC cell line SMMC-7721 to activate autologous T cells. We found that the pulsed DCs stimulated a high level of T-cell proliferation and type I cytokine secretion, and induced HCC-specific CTLs which specifically killed HCC cells by a MHC class I restricted mechanism. Also the hsp70-peptide complexes induced the maturation of DCs by enhanced expression of HLA class II, CD86, and CD83. Our findings provide a new approach for pulsing DCs as tumor vaccines and a rationale for HSP-based vaccination against human HCC.
Human HCC cell line SMMC-7721, human NK cell sensitive cell line K562 and human cervix cell line Hela (American Type Culture Collection) were cultured in RPMI 1640 medium (GIBCO, Invitrogen Corporation, CA, USA) supplemented with 10% heat-inactivated fetal calf serum (FCS) (HyClone, UT, USA), 2 mol/L L-glutamine (GIBCO, Invitrogen Corporation), 100 U/mL penicillin G, 100 μg/mL streptomycin and 250 ng/mL amphotericin B (GIBCOTM, Invitrogen Corporation). Cultures were incubated at 37°C in humidified air with 50 mL/L CO2.
DCs were generated as described with some modifications[16]. Briefly, peripheral blood mononuclear cells (PBMCs) were isolated from healthy donors (Beijing Red Cross Blood Center, China) by Ficoll-Hypaque (1.077 g, Shang Hai Heng Xin Co., China) density gradient centrifugation and cultured in RPMI 1640 medium containing 10% FCS for 2 h. The non -adherent cells were removed for isolation of T cells and the adherent cells were cultured for 6 d in RPMI 1640 medium containing 10% FCS, 1 000 U/mL human GM-CSF (Biosea Biotechnology Co., Beijing, China) and 500 U/mL human IL-4 (Peprotech Inc., NJ, USA). Culture medium and cytokines were refreshed every other day. DCs were harvested from the non-adherent and loosely adherent cells.
T cells were purified by nylon wool column (Polysciences Inc., Warrington, USA) from non-adherent cells according to manufacturer’s instructions. Briefly, non-adherent cells were resuspended in RPMI 1640 medium containing 10% FCS and added to the prepared nylon wool column. After incubating for 1 h at 37°C, the non-adherent cells, which contained mainly T cells, were collected by washing with RPMI 1640 medium containing 10% FCS.
The method for hsp70-peptide complex purification was as described with minor modifications[17]. In brief, SMMC- 7721 tumor cells were harvested by culturing tumor cells in roller bottles and then 10 g tumor cells were suspended in 40 mL (4 volumes) of 30 mmol/L sodium bicarbonate (pH 7.0) and lysed by Dounce homogenization. The homogenate was centrifuged at 100 000 g for 90 min at 4°C and the supernatant obtained. The sample was subjected to column chromatography using Sephadex G25 (Amersham Pharmacia Biotech, NJ, USA) for buffer exchange, affinity chromatography ADP-agarose (Sigma Chemical Co., St. Louis, Missouri, MO, USA), Sephadex G25 for buffer exchange, and ion-exchange chromatography with DEAE-Sepharose (Amersham Pharmacia Biotech, NJ, USA). The eluted proteins were identified by 12% reducing SDS-PAGE followed by silver staining and Western blot with an anti-hsp70 antibody (PharMingen, San Diego, CA, USA). Fractions containing more than 50% hsp70 were stored at -20°C and used for additional experiments. Protein concentration was determined by Bio-Rad protein assay (Bio-Rad Laboratories, Inc., CA, USA). Endotoxin level in the preparations was determined by Limulus amebocyte lysate (LAL) assay (Ocean Biologicals Co., China).
DCs were pulsed with hsp70-peptide complexes purified from SMMC-7721 cells at 37°C for 4 h and then harvested followed by washing with PBS. Autologous T cells isolated by nylon wool column were cocultured with the pulsed DCs in a 96-well flat bottomed culture plate (NUNC, Roskilde, Denmark). Proliferation was determined after 5 d by uptake of tritiated thymidine (Amersham Pharmacia Biotech, Buckinghamshire, UK) measured at 18 h after a pulse of 1 μCi/well.
DCs were pulsed with hsp70-peptide complexes purified from SMMC-7721 cells at 37°C for 4 h. After washing with PBS, DCs were then cocultured with autologous T cells isolated by nylon wool column for 7-10 d in the presence of 20 U/mL human IL-2 (Kexing Co., China) in 24-well culture plate (NUNC, Roskilde, Denmark) . The stimulated T cells were harvested with Ficoll-Hypaque density gradient centrifugation and used as effector cells in the CTL assay using LDH cytotoxicity detection kit (Roche, Indianapolis, USA). SMMC-7721, K562, Hela and autologous DCs pulsed with or without hsp70-peptide complexes were used as target cells in the assay. Briefly, target cells and effector cells were resuspended in assay medium (RPMI 1640 with 1% BSA), and then target cells (1 × 104 cells/well) were cocultured with effector cells at different ratios in a 96-well round bottomed culture plate (NUNC, Roskilde, Denmark) at 37°C. In the indicated experiment, target cells were preincubated with anti-MHC class I antibody (W6/32, PharMingen, San Diego, CA, USA) for 30 min at 37°C before being cocultured with effector cells to test if the cytotoxicity was MHC class I restricted. After 5 h of incubation, the culture plate was centrifuged and the supernatant (100 μL/well) was transferred to another ELISA plate (NUNC, Roskilde, Denmark). LDH detection mixture (100 μL/well) was then added and incubated in the dark for 30 min at room temperature. After adding 50 μL stop solution per well, the absorbance of the samples was measured by ELISA reader (Bio-Rad Laboratories, Inc., CA, USA) at 490 nm with 630 nm as reference wavelength. The spontaneous release of LDH by target cells or effector cells was assessed by incubation of target cells in the absence of effector cells and vice versa. The maximum release of LDH was determined by incubation of target cells in 1% Triton X-100 in assay medium. The percentage of specific cell-mediated cytotoxicity was determined by the following equation: cytotoxicity (%) = [(effector and target mixture-effector spontaneous-target spontaneous)/(maximum-target spontaneous)] × 100.
ELISPOT assay was performed to assess the IFN-γ production of T cells using IFN-γ ELISPOT kit (U-CyTech BV, Utrecht, Netherlands). DCs were pulsed with hsp70-peptide complexes purified from SMMC-7721 cells at 37°C for 4 h. After washing with PBS, DCs were then cocultured with autologous T cells isolated with nylon wool column in the presence of 20 U/mL human IL-2 for 7-10 d. The stimulated T cells (1 × 104/well) as effector cells and irradiated SMMC- 7721 cells (5 × 103/well) as target cells were transferred to ELISPOT plate and incubated for 18 h at 37°C. The IFN-γ secreting spots were developed as described in the IFN- γ ELISPOT kit manual and evaluated in a blind fashion by Dakewe Biotech Co., China, with an automated ELISPOT reader system (Biosys Co. Ltd, Germany).
Flow cytometry DCs were pulsed with hsp70-peptide complexes at 37°C for 4 h and then cultured for another 48 h. The DCs unpulsed or pulsed with hsp70-peptide complex were washed with cold PBS and incubated with murine antibodies directed against human HLA-DR, CD86, CD83 (all from PharMingen, San Diego, USA), for 1 h in ice. After washing with cold PBS, the cells were incubated with FITC conjugated with goat anti-mouse IgG (Zymed Laboratories, Inc., South San Francisco, USA) for 30 min in ice. The cells were then washed with cold PBS and fixed with 2% paraformaldehyde (Sigma). The fluorescence intensity was analyzed by FACS Calibur and the CellQuest software (Becton Dickinson, NJ, USA).
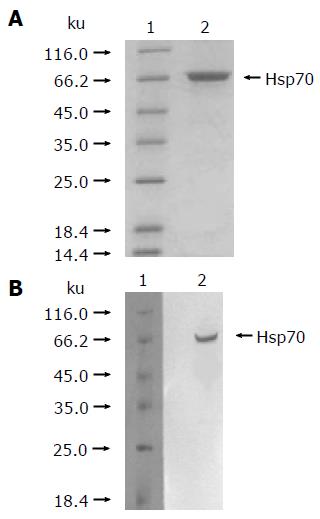
As described in Materials and methods, the hsp70-peptide complexes were purified from HCC SMMC-7721 cells and identified by reduced SDS-PAGE stained with silver and Western blot using an hsp70-specific antibody. The result showed that the purified protein was approximately 72 ku (Figure 1A) and could hybridize with the hsp70-specific antibody (Figure 1B), demonstrating that the obtained protein was hsp70. The purity of the protein preparations was estimated to be >99% by silver-stained SDS-PAGE. The endotoxin level in the preparations was lower than 0.03 EU/μg of hsp70-peptide complexes, as determined by LAL assay.
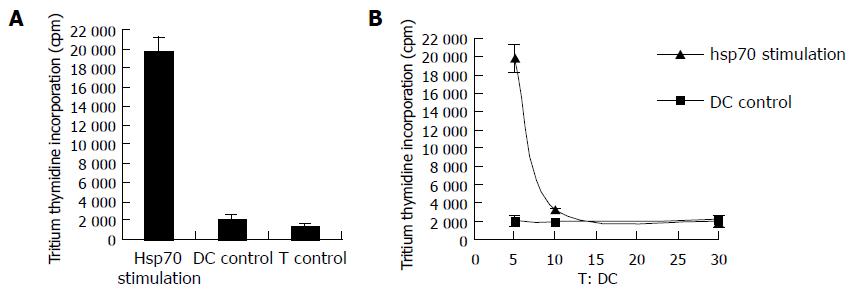
To determine if DCs pulsed with hsp70-peptide complexes derived from SMMC-7721 cells are effective in stimulating autologous T cells, the pulsed DCs were cocultured with autologous T cells. As a control, the T cells were used alone or cocultured with unpulsed DCs. After a 5-d coculture, the T-cell proliferation was tested by uptake of tritiated thymidine. As shown in Figure 2A, DCs pulsed with hsp70-peptide complexes derived from SMMC-7721 cells stimulated autologous T cells to proliferate significantly stronger than did T cells cultured by themselves or cocultured with unpulsed DCs. By contrast, unpulsed DCs exhibited little capacity to stimulate autologous T cells. To further assess the selectivity of this stimulation response, we changed the ratio of DCs:T cells in coculture (Figure 2B). The result showed that the proliferation of T cells cocultured with DCs pulsed with hsp70- peptide complexes was enhanced rapidly by increasing the ratio of DCs:T cells. Contrarily, T-cells coculture with unpulsed DCs proliferated at almost the same level despite an increase in the ratio of DCs:T cells. These results demonstrate that DCs pulsed with hsp70 -peptide complexes are capable of specifically stimulating T-cell responses.
Cell-mediated immunity, which is particularly important in suppressing tumors, is characterized by induction of CTLs and production of type I cytokines. To explore whether DCs pulsed with hsp70-peptide complexes derived from SMMC-7721 cells are capable of generating SMMC-7721-specific CTLs and inducing type I cytokine secretion, we examined cytotoxic activities and IFN-γ production of autologous T cells cocultured with DCs. DCs pulsed with hsp70-peptide complexes were cocultured with autologous T cells for 7- 10 d in the presence of 20 U/mL human IL-2 and then the T cells were isolated by density gradient centrifugation for cytotoxicity testing and type I cytokine secretion assessing. As a control, the T cells were used alone or cocultured with unpulsed DCs.
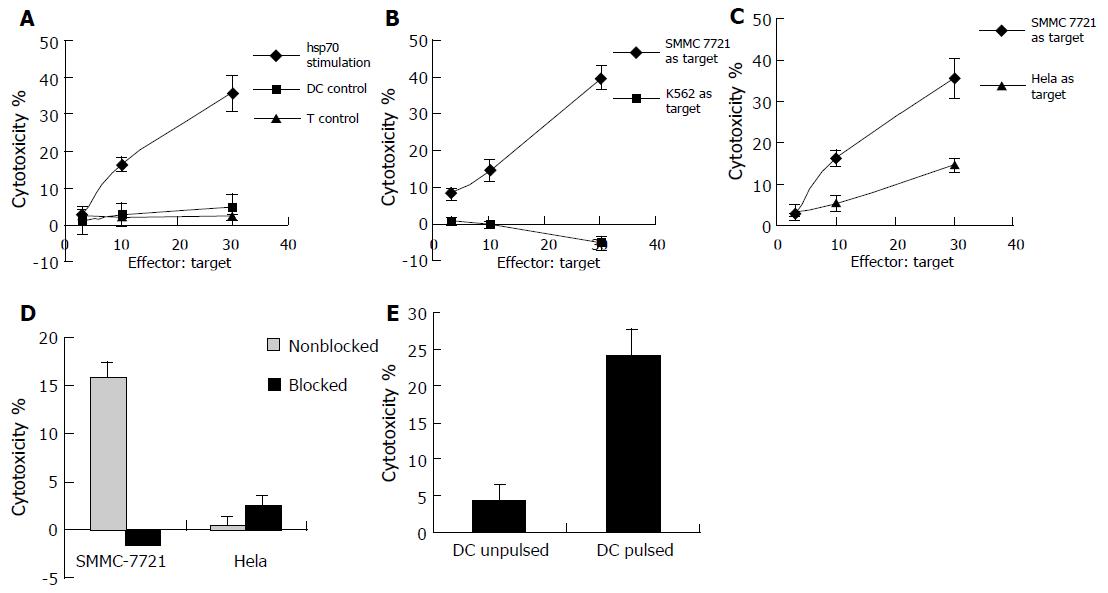
The result of cytotoxicity testing showed that T cells cocultured with unpulsed DCs or by themselves failed to exhibit evident cytotoxicity against SMMC -7721 cells, but significantly, T cells cocultured with DCs pulsed with hsp70-peptide complexes were capable of killing SMMC-7721 cells at high levels (Figure 3A). To test if the lysis against SMMC-7721 was due to the presence of NK cells, the NK cell-sensitive cell line K562 was used as target cell in the cytotoxicity assay (Figure 3B). The result showed that the cytotoxicity against K562 cells was very little, much lower than that against SMMC-7721 cells, indicating that it was not NK cells but rather CTLs that killed SMMC-7721 cells.
To further prove that CTLs killed the SMMC-7721 cells and to define the specificity of the CTLs generated by the coculture of T cells with autologous DCs pulsed with hsp70-peptide complexes, we compared the cytotoxicity of the T-cell against SMMC-7721 cells and another human cervix cell line Hela cells (Figure 3C), and assessed if the cytotoxicity was MHC class I restricted (Figure 3D). As shown in Figure 3C, T cells stimulated by DCs pulsed with hsp70-peptide complexes showed significant cytotoxicity against SMMC-7721 cells, whereas they exhibited little lysis against Hela cells. As shown in Figure 3D, preincubation of SMMC-7721 cells with anti-MHC class I antibody resulted in abrogation of tumor cell lysis; however, the anti-MHC class I antibody had little if any effect on lysis of Hela cells, demonstrating that the cytotoxicity against SMMC-7721 cells was MHC class I restricted and therefore due to CD8+ T cells which directed against tumor-specific antigens. The quite weak lysis against Hela cells may result from non-specific killing activity. These results demonstrated that CTLs induced by autologous DCs pulsed with hsp70-peptide complexes were specific to SMMC-7721 cells from which hsp70-peptide complexes were derived.
To exclude the possibility that the SMMC-7721 specific cytotoxicity was due to allogeneic response, autologous DCs pulsed or unpulsed with hsp70-peptide complexes were used as target cells in CTL assay. As shown in Figure 3E, the CTLs induced by DCs pulsed with hsp70-peptide complexes exhibited significant cytotoxicity against DCs pulsed with hsp70-peptide complexes which expressed tumor antigens on their surface, whereas little cytotoxicity was exhibited against unpulsed DCs which did not express tumor antigens. The result confirmed that the cytotoxicity against SMMC-7721 was a tumor-specific response rather than an allogeneic response.
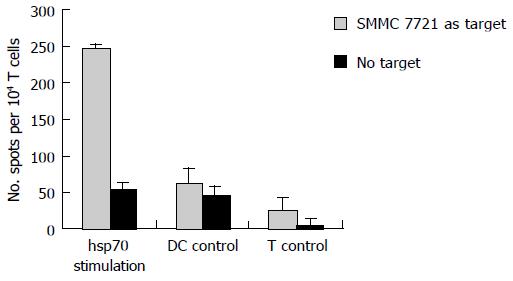
In addition to the cytotoxicity test, we also examined if DCs pulsed with hsp70-peptide complexes could induce autologous T cells to secret IFN-γ . As shown in Figure 4, T cells cocultured with DCs pulsed with hsp70-peptide complexes secreted a high level of IFN-γ against SMMC-7721; however, T cells cocultured with unpulsed DCs or by themselves secreted very little IFN-γ . Consistent with the CTL testing result, this finding suggested that DCs pulsed with hsp70-peptide complexes efficiently induce SMMC-7721 specific CTLs, which secrete type I cytokines at a high level.
These findings illuminate that DCs pulsed with hsp70-peptide complexes are very effective in inducing functional CTLs which direct to tumor cells from which hsp70-peptide complexes are isolated and secrete type I cytokine at high levels.
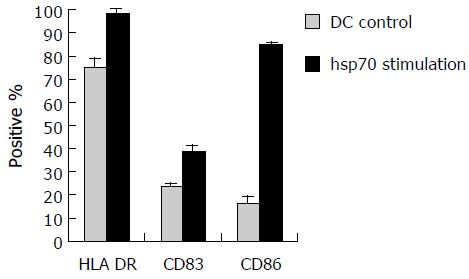
To examine the effect of hsp70-peptide complexes derived from SMMC-7721 cells on DCs, immature DCs generated by culturing human PBMCs in the presence of human GM-CSF and IL-4 for 6 d, were incubated with hsp70-peptide complexes for 4 h at 37°C and then cultured for 48 h. The expression level of HLA-DR, CD86, and CD83 was determined by flow cytometry. The result showed that hsp70-peptide complexes promoted upregulation of HLA-DR, co-stimulation molecule CD86 and maturation marker CD83 (Figure 5), indicating that hsp70-peptide complexes induced maturation of DCs. This maturation effect of hsp70-peptide complexes on DCs suggests that they can activate DCs effectively.
Cancer, which is difficult to cure completely using conventional treatment such as surgery, radiation or chemotherapy, has become one of the most common causes of death in industrialized societies[3]. Immunotherapy, which utilizes the immune response in combating cancer, seems to be a potentially effective approach for treatment of cancer. Numerous vaccination approaches have been explored and developing more effective anti-tumor vaccines has become one of the major goals in tumor immunotherapy.
It is commonly accepted that tumor rejection is mediated by lymphocytes, and most notably by CTLs. Therefore, activating tumor -specific CTLs to kill tumor cells is considered to be one of the most important events in tumor immunotherapy[18]. T-cell activation requires two signals. Signal one is generated when TCR interacts with the MHC-peptide complex and signal two is provided by costimulatory molecules expressed by professional APCs. Most tumors do not express costimulatory molecules and thus fail to activate tumor-specific T lymphocytes[19]. The HCC cell line SMMC-7721 expresses MHC class I molecules, but does not express costimulatory molecules, such as CD80 or CD86 (data not shown). So to generate tumor-specific CTLs, it is essential to find a good tumor antigen source and then present them in the context of costimulatory molecules.
The features of HSPs suggest that they are very potent tumor antigen sources for pulsing DCs to stimulate anti-tumor response. Three features characterize HSPs as a tumor antigen source: first, they can induce multiple anti-tumor immune responses without prior identification of tumor antigens; second, they can chaperone tumor antigens associated with them and present them with high efficiency through specific receptors on DCs; third, they can induce phenotypical and functional maturation of DCs. In the present study, we used hsp70-peptide complexes derived from HCC cell line SMMC-7721 as the tumor antigen source to pulse DCs, which are known to be critical activators of T-cell responses. Various studies suggest that hsp70 has immunotherapeutic potential as hsp70 purified from malignant and virally infected cells can transfer and deliver antigenic peptides to APCs to elicit peptide-specific immunity[20]. We demonstrated that DCs pulsed with hsp70-peptide complexes derived from human HCC cell line SMMC-7721 stimulated significant autologous T-cell proliferation and that unpulsed DCs had little stimulatory effect (Figure 2). More importantly, we proved that DCs pulsed with hsp70 -peptide complexes were able to induce tumor cell-specific CTLs with high efficiency and specificity (Figure 3) and the generated CTLs could secrete a high level of type I cytokine (Figure 4). Our results demonstrated that the cytotoxicity against SMMC-7721 cells was due to the presence of CTLs but not NK cells because NK cell-sensitive cell line K562 could not be killed (Figure 3B). In addition, we tested the phenotype of the cytotoxic cells using T-cell lineage marker CD3, NK cell lineage marker CD56 and NK- T-cell lineage marker CD56+CD3 by flow cytometry (data not shown). The result showed that most of the cytotoxic cells were T cells and there were very few NK cells or NK-T cells. Also we demonstrated that the generated CTLs exhibited specific cytotoxicity against SMMC-7721 cells (Figure 3C) and the cytotoxicity was MHC class I restricted (Figure 3D). Furthermore we excluded the possibility that the induced anti-tumor response was an allogeneic response (Figure 3E). The result indicated that the generated CTLs exhibited significant cytotoxicity against autologous DCs pulsed with hsp70-peptide complexes, but little lysis against unpulsed DCs, demonstrating that the CTLs were directed against tumor-specific antigens expressed on DCs rather than allo-antigens. This result also implied that DCs took up hsp70-peptide complexes, and then processed and presented tumor-specific antigens in the MHC class I pathway, making them available for CTL recognition. Our findings indicated that the SMMC-7721 specific antigens chaperoned by hsp70 had been functionally presented to T cells through the active interaction of hsp70 and DCs. The implication of the original studies was that immunization of HSPs was able to generate anti-tumor CTLs via cross-priming by transferring the antigenic peptides with which they were associated into the MHC class I antigen presenting pathway within APCs, a pathway usually restricted to antigens derived from the cytoplasm of the APCs[21]. The mechanism of receptor-mediated endocytosis contributes to the high efficiency of antigen transfer[10,22]. More recently, different receptors involved in the capacity of hsp70 to induce peptide-specific immunity have been identified[11,23]. Consistent with these observations, our results indicated that hsp70 was a potent adjuvant for presenting tumor-associated antigens and then inducing specific anti-tumor immune responses.
Activation of DCs is a key step in the stimulation of tumor-specific CTLs. As professional APCs, with a potent capacity to present antigens and activate naive T cells, DCs can both initiate and modulate immune responses[24]. In the present study, we showed that hsp70-peptide complexes derived from HCC cell line SMMC-7721 induced maturation of allogeneic DCs by enhancing the expression of HLA class II, costimulatory molecule CD86, and maturation maker CD83. The matured DCs were able to stimulate high levels of autologous T- cell proliferation and type I cytokine secretion, and to induce tumor-specific CTLs with high efficiency. The results indicate that hsp70-peptide complexes derived from HCC cell line SMMC-7721 have the capacity to induce human DCs maturation phenotypically and functionally. The phenotypical and functional maturation is critical for DCs to efficiently activate immune responses[25].
Consistent with previous reports[26] , MHC matching between the SMMC-7721 cells used as source of hsp70-peptide complexes and the responding T cells was not required, suggesting that the peptides complexed by hsp70 were not restricted to the MHC haplotype of the donor cells and hsp70 -peptide complexes were able to induce tumor-specific CTLs across MHC barriers by active interaction with DCs.
In conclusion, our present study demonstrated varied attributes of hsp70 which included chaperoning a broad array of antigenic peptides, delivering them into MHC class I antigen presentation pathway within DCs via cross-priming through receptor-mediated interaction, and activating DCs phenotypically and functionally. These properties of hsp70 suggest that hsp70-peptide complexes are appealing antigen sources for immunization against tumors and infectious diseases. In this study, we combined the characteristics of DCs and hsp70 for stimulating anti-tumor immune responses and offered a new kind of anti-tumor immunotherapeutic strategy against human HCC. Our results suggest that DCs pulsed with hsp70-peptide complexes derived from human HCC cells can be used as potential vaccines in anti -tumor immunotherapy, as these pulsed DCs are capable of inducing multiple CTLs efficiently without the need for identifying tumor-specific antigens. In the present study, we used allogeneic rather than autologous HCC cells to isolate hsp70 -peptide complexes for pulsing DCs derived from healthy donors. We hope to apply this approach to HCC patients in future clinical research.
We thank the Beijing Red Cross Blood Center for supplying the blood samples. We also thank the FACS laboratory of the Medical School of Peking University for performing the FACS analysis.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Liu BB, Ye SL, He P, Liu YK, Tang ZY. MAGE-1 and related MAGE gene expression may be associated with hepatocellular carcinoma. J Cancer Res Clin Oncol. 1999;125:685-689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Takayama T, Sekine T, Makuuchi M, Yamasaki S, Kosuge T, Yamamoto J, Shimada K, Sakamoto M, Hirohashi S, Ohashi Y. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet. 2000;356:802-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 630] [Cited by in RCA: 653] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 3. | Pandey M, Mathew A, Nair MK. Cancer vaccines: a step towards prevention and treatment of cancer. Eur J Surg Oncol. 1999;25:209-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 4. | Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10867] [Cited by in RCA: 10725] [Article Influence: 397.2] [Reference Citation Analysis (0)] |
| 5. | Pockley AG. Heat shock proteins as regulators of the immune response. Lancet. 2003;362:469-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 501] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 6. | Fujihara SM, Nadler SG. Intranuclear targeted delivery of functional NF-kappaB by 70 kDa heat shock protein. EMBO J. 1999;18:411-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Wallin RP, Lundqvist A, Moré SH, von Bonin A, Kiessling R, Ljunggren HG. Heat-shock proteins as activators of the innate immune system. Trends Immunol. 2002;23:130-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 427] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 8. | Janetzki S, Palla D, Rosenhauer V, Lochs H, Lewis JJ, Srivastava PK. Immunization of cancer patients with autologous cancer-derived heat shock protein gp96 preparations: a pilot study. Int J Cancer. 2000;88:232-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 9. | Tamura Y, Peng P, Liu K, Daou M, Srivastava PK. Immunotherapy of tumors with autologous tumor-derived heat shock protein preparations. Science. 1997;278:117-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 488] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 10. | Arnold-Schild D, Hanau D, Spehner D, Schmid C, Rammensee HG, de la Salle H, Schild H. Cutting edge: receptor-mediated endocytosis of heat shock proteins by professional antigen-presenting cells. J Immunol. 1999;162:3757-3760. [PubMed] |
| 11. | Basu S, Binder RJ, Ramalingam T, Srivastava PK. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity. 2001;14:303-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 844] [Cited by in RCA: 796] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 12. | Singh-Jasuja H, Scherer HU, Hilf N, Arnold-Schild D, Rammensee HG, Toes RE, Schild H. The heat shock protein gp96 induces maturation of dendritic cells and down-regulation of its receptor. Eur J Immunol. 2000;30:2211-2215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Singh-Jasuja H, Toes RE, Spee P, Münz C, Hilf N, Schoenberger SP, Ricciardi-Castagnoli P, Neefjes J, Rammensee HG, Arnold-Schild D. Cross-presentation of glycoprotein 96-associated antigens on major histocompatibility complex class I molecules requires receptor-mediated endocytosis. J Exp Med. 2000;191:1965-1974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 269] [Cited by in RCA: 263] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 14. | Suto R, Srivastava PK. A mechanism for the specific immunogenicity of heat shock protein-chaperoned peptides. Science. 1995;269:1585-1588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 655] [Cited by in RCA: 615] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 15. | Cho BK, Palliser D, Guillen E, Wisniewski J, Young RA, Chen J, Eisen HN. A proposed mechanism for the induction of cytotoxic T lymphocyte production by heat shock fusion proteins. Immunity. 2000;12:263-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 103] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 16. | Thurner B, Röder C, Dieckmann D, Heuer M, Kruse M, Glaser A, Keikavoussi P, Kämpgen E, Bender A, Schuler G. Generation of large numbers of fully mature and stable dendritic cells from leukapheresis products for clinical application. J Immunol Methods. 1999;223:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 383] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 17. | Srivastava PK, Jaikaria NS. Methods of purification of heat shock protein-peptide complexes for use as vaccines against cancers and infectious diseases. Methods Mol Biol. 2001;156:175-186. [PubMed] |
| 18. | Hanson HL, Donermeyer DL, Ikeda H, White JM, Shankaran V, Old LJ, Shiku H, Schreiber RD, Allen PM. Eradication of established tumors by CD8+ T cell adoptive immunotherapy. Immunity. 2000;13:265-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 269] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 19. | Cavallo F, Martin-Fontecha A, Bellone M, Heltai S, Gatti E, Tornaghi P, Freschi M, Forni G, Dellabona P, Casorati G. Co-expression of B7-1 and ICAM-1 on tumors is required for rejection and the establishment of a memory response. Eur J Immunol. 1995;25:1154-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 94] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Ciupitu AM, Petersson M, Kono K, Charo J, Kiessling R. Immunization with heat shock protein 70 from methylcholanthrene-induced sarcomas induces tumor protection correlating with in vitro T cell responses. Cancer Immunol Immunother. 2002;51:163-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Srivastava PK, Menoret A, Basu S, Binder RJ, McQuade KL. Heat shock proteins come of age: primitive functions acquire new roles in an adaptive world. Immunity. 1998;8:657-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 376] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 22. | Binder RJ, Harris ML, Ménoret A, Srivastava PK. Saturation, competition, and specificity in interaction of heat shock proteins (hsp) gp96, hsp90, and hsp70 with CD11b+ cells. J Immunol. 2000;165:2582-2587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 93] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Becker T, Hartl FU, Wieland F. CD40, an extracellular receptor for binding and uptake of Hsp70-peptide complexes. J Cell Biol. 2002;158:1277-1285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 286] [Cited by in RCA: 259] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 24. | Liu YJ. Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell. 2001;106:259-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 747] [Cited by in RCA: 720] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 25. | Wan T, Zhou X, Chen G, An H, Chen T, Zhang W, Liu S, Jiang Y, Yang F, Wu Y. Novel heat shock protein Hsp70L1 activates dendritic cells and acts as a Th1 polarizing adjuvant. Blood. 2004;103:1747-1754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 84] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Castelli C, Ciupitu AM, Rini F, Rivoltini L, Mazzocchi A, Kiessling R, Parmiani G. Human heat shock protein 70 peptide complexes specifically activate antimelanoma T cells. Cancer Res. 2001;61:222-227. [PubMed] |