Published online Sep 21, 2005. doi: 10.3748/wjg.v11.i35.5565
Revised: January 23, 2005
Accepted: January 26, 2005
Published online: September 21, 2005
AIM: To investigate the presence of HBsAg, HBcAg, and HBV DNA in ovarian tissues from patients with HBV infection.
METHODS: HBsAg and HBcAg were examined in ovarian biopsy tissues from 26 patients with HBV infection by immunocytochemistry, and HBV DNA was detected in ovarian tissues by PCR.
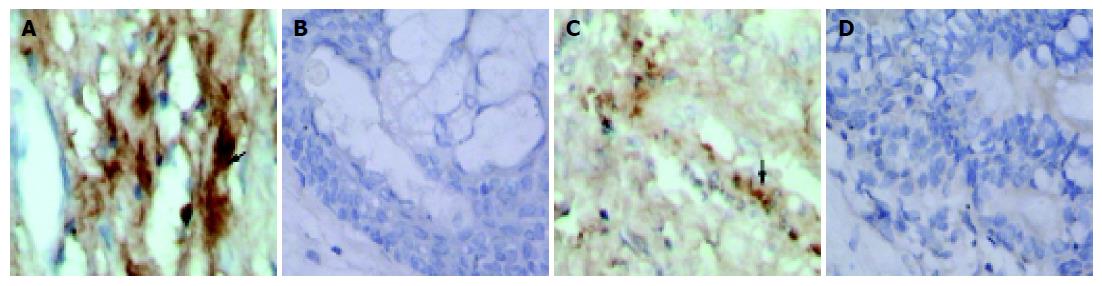
RESULTS: HBsAg and HBcAg were present with the same positive rate of 34.6% (9/26). The total positive rate was 46.2% (12/26). HBsAg and HBcAg were positive in 6 (23.1%) of the 26 patients. Brown positive particles were diffusely distributed in ovarian cells. The positive rate of HBV DNA was 58.3% (7/12).
CONCLUSION: HBsAg, HBcAg, and HBV DNA can be detected in ovarian tissues from patients with HBV infection. The presence of HBsAg and HBcAg in ovarian tissues does not correlate with the HBV markers in serum.
- Citation: Chen LZ, Fan XG, Gao JM. Detection of HBsAg, HBcAg, and HBV DNA in ovarian tissues from patients with HBV infection. World J Gastroenterol 2005; 11(35): 5565-5567
- URL: https://www.wjgnet.com/1007-9327/full/v11/i35/5565.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i35.5565
The prevalence of hepatitis B is high in China. The overall positive rate of serum HBsAg is about 10%. Hepatitis B virus (HBV) infection can cause acute hepatitis, chronic hepatitis, hepatocirrhosis and hepatocellular carcinoma[1,2]. HBV is not a kind of virus that locates strictly in the liver cells. HBV infection may cause either pathological hepatic lesion or extra-hepatic lesion. It has been well documented that the extra-hepatic lesion due to acute hepatitis is temporary, and that due to chronic hepatitis is associated with persistent HBV infection and draws more attention of researchers. Animal experiments have confirmed that HBV can exist and replicate in various organs and tissues[3,4]. With the development of molecular biology, HBV can be detected in patients with HBV infection outside the liver, such as in peripheral blood mononuclear cells, tissues or cells of pancreas, spleen, skin, kidney, etc.[5]. Moreover, it has been shown to be present in blood, saliva, semen, vagina secretion, sweat, breast milk, tear, and urine[6].
Although some studies on the infection and location of HBV in extra-hepatic tissues have been carried out and a few papers have been published at home or abroad[3-5], whether HBV replicates outside the liver after its infection is still controversial. The location and distribution of HBV in extra-hepatic tissues or cells are not consistent, and the pathologic lesions caused by the extra-hepatic infection of HBV and their pathogenesis are not fully understood. Whether HBV exists in ovarian tissues is unknown. If we can demonstrate this, then it is reasonable to assume that vertical transmission may be due to HBV transmitting from ovary to ovum and fetus. In this study, we investigated the presence of HBsAg, HBcAg, and HBV DNA in 26 ovarian biopsy specimens using immunocytochemistry and PCR in order to analyze and interpret the mechanism and significance of HBV infection in ovarian tissues.
We reviewed the medical history at the Department of Pathology, Xiangya Hospital, Central South University and found 170 patients who had ovarian biopsy from June 2002 to June 2003. Among them, 26 patients had positive serum HBV markers. Seven out of the twenty-six patients were positive for HBsAg, and the other 19 patients had two or more HBV markers in serum. Paraffin-embedded ovarian tissues from these 26 patients were enrolled for further analysis. The age of the 26 patients ranged from 2 mo to 71 years, with an average of 40 years. Five patients were diagnosed as ovarian cyst, and 21 patients as ovarian cancer.
The presence of HBsAg and HBcAg was examined in ovarian biopsy tissues from the 26 patients by immunocy-tochemistry (streptavidin-peroxidase-biotin) according to the manufacturer’s instructions. The main steps were as follows. Cover slips were dewaxed with xylene, and dehydrated in alcohol, washed thrice with PBS for 5 min each time. Endogenous peroxidase activity was quenched by incubating cover slips with 50 µL peroxide for 10 min, and washed with PBS. Samples were blocked by incubating cover slips with 50 µL goat serum for 30 min, washed with PBS and incubated with primary antibody for 60 min at room temperature, then washed with PBS and incubated with biotin-conjugated secondary antibody for 10 min at room temperature, washed again with PBS and incubated with streptavidin-peroxidase for 10 min at room temperature, washed with PBS and incubated with DAB for 5 min, counterstained with hematoxylin, dehydrated in alcohol and xylene. The coverslips were permanently mounted and observed under light microscope. Brown signals were considered as positive result.
When primary antibody was replaced by PBS, the result was negative.
Ovarian tissues from patients without HBV infection were used as negative control.
Liver tissues from patients with hepatitis B were used as positive control.
Mouse anti-HBsAg, mouse anti-HBcAg, and SP kit were purchased from Zhongshan Biological Technology Limited Company, Beijing.
Paraffin-embedded ovarian tissues were digested with proteinase K, and HBV DNA was isolated using phenol and chloroform. Primer sequences were designed by us using Primer 3 software: forward primer, 5’-TCGGAAATACACCTCCTTTCCATGG-3’ (HBV genome 1 353-1 377); reverse primer, 5’-GCCTCAA GGTCGGT-CGTTGACA-3’ (HBV genome 1 702-1 681). The length of PCR product was 350 bp. Liver tissue DNA from patients with hepatitis B and ovarian tissue DNA from patients without HBV infection were used as positive control and negative control respectively. Thirty cycles of DNA amplification were performed in 50 μL PCR reaction mixture. The condition of each cycle was denaturation at 94 °C for 30 s, primer annealing at 55 °C for 30 s, and elongation at 72 °C for 30 s, followed by a final 10-min elongation at 72 °C. DNA marker, proteinase K, Taq DNA polymerase and dNTP were purchased from TaKaRa Company, Dalian. Primer was synthesized by BioAsia Company, Shanghai.
The results of immunocytochemistry indicated that HBsAg and HBcAg were present in ovarian tissues (Figures 1). The positive rate of HBsAg and HBcAg in ovarian tissue was 34.6% (9/26), respectively. The total positive rate was 46.2% (12/26). HBsAg and HBcAg were positive in 6 (23.1%) of the 26 cases. Brown positive particles were predominantly within the ovarian cells, depositing linearly or in clusters. According to HBV markers in serum, the 26 patients were divided into two groups: patients with one indicator positive and patients with two or more positive indicators. The positive rate of HBsAg and HBcAg in ovarian tissues of these two groups showed no statistical difference (P>0.05) as listed in Table 1.
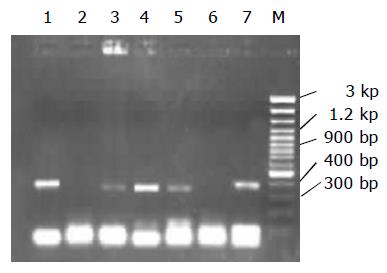
HBV DNA was examined in ovarian tissues from the 12 patients with positive immunocytochemical results. The positive rate of HBV DNA was 58.3% (7/12), as shown in Figure 2.
Whether HBV can locate and replicate in ovarian tissue is not clear. This is the first report to detect HBsAg, HBcAg, and HBV DNA in ovarian tissues by immunocytochemistry and PCR. Our data indicate the positive rate of HBsAg and HBcAg was 34.6% (9/26) respectively, and the presence of HBsAg and HBcAg in ovarian tissues does not correlate with the HBV markers in serum.
PCR is a technique for quickly cloning a particular piece of DNA in vitro. The essential steps include thermal denaturation of the double-stranded molecules, the primers binding to their complementary template, and extension of the primers by enzymatic synthesis with DNA polymerase. The reaction is efficient, specific, and extremely sensitive[7-9]. Using PCR to assay clinical specimens has become a common method in the diagnosis of infectious diseases. HBV DNA is present in ovarian tissues with a positive rate of 58.3%. Our data provide evidence for extra-hepatic infection of HBV.
It is not clear what pathologic changes might be induced in ovary by HBV infection. An early study[10] reported that HBV can induce immune damage, even cell carcinogenesis through antigen expression in cells or integration to cell genome. In our research, all the samples were collected from patients with ovarian tumor. Whether HBV infection in ovary relates to ovarian tumor needs further study. HBV extra-hepatic infection plays a role in the preservation of the virus, but extra-hepatic tissues and cells with HBV infection often escape immune damage without obvious pathologic changes because of the small amount of viruses and the incomplete protein expression in extra-hepatic infection.
Extra-hepatic HBV infection should be targeted as liver pathologic changes in the treatment of hepatitis B. Since our study indicated the presence of HBV in ovary, and HBV might be possible to replicate in ovary, it is reasonable to assume that vertical transmission is due to HBV transmitting from ovary to ovum and fetus. There are three classical mechanisms underlying HBV transmission from mother to child[11,12]. (1) HBV transmit to the fetus through placenta, namely intrauterine infection, which has been confirmed by studies based on newborn, abortus and germ cells. (2) If there is a small cut in the skin of the fetus, and the cut is contaminated by the mother’s blood or vaginal secretion in the process of childbearing, HBV infection may occur. This is the main type of vertical transmission. (3) Mother to child horizontal transmission is the main transmission route via breast milk feeding. Further studies should be focused on the transmission routes of HBV in order to control and prevent hepatitis B.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Lansang MA. Epidemiology and control of hepatitis B infection: a perspective from the Philippines, Asia. Gut. 1996;38 Suppl 2:S43-S47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Maupas P, Chiron JP, Barin F, Coursaget P, Goudeau A, Perrin J, Denis F, Mar ID. Efficacy of hepatitis B vaccine in prevention of early HBsAg carrier state in children. Controlled trial in an endemic area (Senegal). Lancet. 1981;1:289-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 144] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Hosoda K, Omata M, Uchiumi K, Imazeki F, Yokosuka O, Ito Y, Okuda K, Ohto M. Extrahepatic replication of duck hepatitis B virus: more than expected. Hepatology. 1990;11:44-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Chen LB, Chen PL, Wang GL, Song XT, Li YF, Liu CY, Jia KM. Ultrastructural study on extrahepatic infection of duck hepatitis B virus in ducks. Chin Med J (Engl). 1992;105:212-216. [PubMed] |
| 5. | Lin CY. Hepatitis B virus deoxyribonucleic acid in kidney cells probably leading to viral pathogenesis among hepatitis B virus associated membranous nephropathy patients. Nephron. 1993;63:58-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Boag F. Hepatitis B: heterosexual transmission and vaccination strategies. Int J STD AIDS. 1991;2:318-324. [PubMed] |
| 7. | Gao H, Kong J, Wang JY, Liu HY, Wen J. The study on hepatitis B maternal-infant longitudinal transmission by nested PCR technology. Zhongguo Yike Daxue Xuebao. 1998;27:59-62. |
| 8. | Ou ZS, Song WG, Yin XM, Chen XL, Gao LY, Ding CF. Study of application of PCR technique to the diagnosis of vertical transmission of HBV. Taishan Yixueyuan Xuebao. 1998;19:10-13. |
| 9. | Feinman SV, Berris B, Guha A, Sooknanan R, Bradley DW, Bond WW, Maynard JE. DNA: DNA hybridization method for the diagnosis of hepatitis B infection. J Virol Methods. 1984;8:199-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Chu CM, Liaw YF. Intrahepatic distribution of hepatitis B surface and core antigens in chronic hepatitis B virus infection. Hepatocyte with cytoplasmic/membranous hepatitis B core antigen as a possible target for immune hepatocytolysis. Gastroenterology. 1987;92:220-225. [PubMed] |
| 11. | Jiang JX. Advance on the study of vertical transmission of HBV. Zhongguo Xiandai Yixue Zazhi. 2001;11:26-27. |
| 12. | Zhu DY, Huang HY, Zheng JS. The transmission routes of mother to child transmission of HBV and its prevention. Zhonghua Huli Zazhi. 2001;36:209-210. |