Published online Sep 21, 2005. doi: 10.3748/wjg.v11.i35.5485
Revised: March 21, 2005
Accepted: March 24, 2005
Published online: September 21, 2005
AIM: To investigate the functional, morphological changes of the gut barrier during the restitution process after hemorrhagic shock, and the regional differences of the large intestine and small intestine in response to ischemia/reperfusion injury.
METHODS: Forty-seven Sprague-Dawley rats with body weight of 250-300 g were divided into two groups: control group (sham shock n = 5) and experimental group (n = 42). Experimental group was further divided into six groups (n = 7 each) according to different time points after the hemorrhagic shock, including 0th h group, 1st h group, 3rd h group, 6th h group, 12th h group and 24th h group. All the rats were gavaged with 2 mL of suspension of lactulose (L) (100 mg/2 mL) and mannitol (M) (50 mg/each) at the beginning and then an experimental rat model of hemorrhagic shock was set up. The specimens from jejunum, ileum and colon tissues and the blood samples from the portal vein were taken at 0, 1, 3, 6, 12 and 24 h after shock resuscitation, respectively. The morphological changes of the intestinal mucosa, including the histology of intestinal mucosa, the thickness of mucosa, the height of villi, the index of mucosal damage and the numbers of goblet cells, were determined by light microscope and/or electron microscope. The concentrations of the bacterial endotoxin lipopolysaccharides (LPS) from the portal vein blood, which reflected the gut barrier function, were examined by using Limulus test. At the same time point, to evaluate intestinal permeability, all urine was collected and the concentrations of the metabolically inactive markers such as L and M in urine were measured by using GC-9A gas chromatographic instrument.
RESULTS: After the hemorrhagic shock, the mucosal epithelial injury was obvious in small intestine even at the 0th h, and it became more serious at the 1st and the 3rd h. The tissue restitution was also found after 3 h, though the injury was still serious. Most of the injured mucosal restitution was established after 6 h and completed in 24 h. Two distinct models of cell death-apoptosis and necrosis-were involved in the destruction of rat intestinal epithelial cells. The number of goblet cells on intestinal mucosa was reduced significantly from 0 to 24 h (the number from 243±13 to 157±9 for ileum, 310±19 to 248±18 for colon; r = -0.910 and -0.437 respectively, all P<0.001), which was the same with the large intestine, but the grade of injury was lighter with the values of mucosal damage index in 3 h for jejunum, ileum, and colon being 2.8, 2.6, 1.2, respectively. The mucosal thickness and the height of villi in jejunum and ileum diminished in 1 h (the average height decreased from 309±24 to 204±23 µm and 271±31 to 231±28 µm, r = -0.758 and -0.659, all P<0.001; the thickness from 547±23 to 418±28 µm and 483±45 to 364±35 µm, r = -0.898 and -0.829, all P<0.001), but there was no statistical difference in the colon (F = 0.296, P = 0.934). Compared with control group, the urine L/M ratio and the blood LPS concentration in the experimental groups raised significantly, reaching the peak in 3-6 h (L/M: control vs 3 h vs 6 h was 0.029±0.09 vs 0.063±0.012 vs 0.078±0.021, r = -0.786, P<0.001; LPS: control vs 3 h vs 6 h was 0.09±0.021 vs 0.063±0.012 vs 0.25±0.023, r = -0.623, P<0.001), and it kept increasing in 24 h.
CONCLUSION: The gut barrier of the rats was seriously damaged at the early phase of ischemic reperfusion injury after hemorrhagic shock, which included the injury and atrophy in intestinal mucosa and the increasing of intestinal permeability. Simultaneously, the intestinal mucosa also showed its great repairing potentiality, such as the improvement of the intestinal permeability and the recovery of the morphology at different phases after ischemic reperfusion injury. The restitution of gut barrier function was obviously slower than that of the morphology and there was no direct correlation between them. Compared with the small intestine, the large intestine had stronger potentiality against injury. The reduction of the amount of intestinal goblet cells by injury did not influence the ability of intestinal mucosal restitution at a certain extent and it appeared to be intimately involved in the restitution of the epithelium.
- Citation: Chang JX, Chen S, Ma LP, Jiang LY, Chen JW, Chang RM, Wen LQ, Wu W, Jiang ZP, Huang ZT. Functional and morphological changes of the gut barrier during the restitution process after hemorrhagic shock. World J Gastroenterol 2005; 11(35): 5485-5491
- URL: https://www.wjgnet.com/1007-9327/full/v11/i35/5485.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i35.5485
In recent years, it has been made clear that the intestinal tract is one of the earliest organs involved in ischemia-reperfusion injury after hemorrhagic shock. Owing to the increased intestinal permeability with gut barrier injury, the bacteria and lipopolysaccharides (LPS) can enter the systemic circulation through the portal vein and the mesenteric lymph, and result in sepsis and multiple organ dysfunction syndrome (MODS)[1-3]. The intestinal tract, therefore, is regarded as “initiator”of MODS and its potential pathogenicity has been given more attention. Although the recent focus has been on the study of intestinal barrier function, there are a lot of problems to be solved and the exact mechanism about gut barrier function is uncertain[4,5]. The object of this study, based on the model of intestinal ischemia-reperfusion injury in the early phases after hemorrhagic shock, was to observe the intestinal mucosal changes in all process from injury to restitution after hemorrhagic shock and collect more evidences on the function of gut barrier and its influencing factors.
To begin with, 47 adult and healthy Sprague-Dawley rats with body weight of 250-300 g were used (supplied by Experimental Animal Center, Sun Yat-Sen Medical College, Sun Yat-Sen University) and were gavaged with suspension of lactulose (L) (100 mg/each) and mannitol (M) (50 mg/each) dissolved in 2 mL water (Sigma Chemical Company, USA). All rats were randomly divided into two groups: control group (sham shock n = 5) and experimental group (n = 42 each). According to the different time points of the shock resuscitation, the experimental group was further divided into six groups (n = 7 each), namely 0th h group, 1st h, 3rd h, 6th h, 12th h and 24th h group.
Animals were subjected to hemorrhagic shock as previously described[6]. Briefly, the rats were anesthetized with an intraperitoneal injection of 1 200 mg/kg of urethane. A 1.5 cm incision was made, and the common carotid artery and jugular vein were catheterized. The rats were bled to a mean arterial pressure of 40 mm Hg and kept for 60 min and the shock ended, when the initially shed blood volume and partes aequales of saline were reinfused. Following the termination of shock, the animals were killed at 0, 1, 3, 6, 12 and 24 h time points, respectively. Several segments were taken from jejunum, ileum and colon respectively and rinsed with ice-cold normal saline, fixed in 20% buffered formalin and 3% glutaraldehyde. Meanwhile, 1.5 mL of portal vein blood was drawn from each rat, centrifuged and stored at -80 °C. All urine, except for that of 0th h group, were collected and stored at -80 °C. The control rats were only anesthetized and catheterized, not bled.
Tissue samples were prepared for histological examination of lesions. The resected segments of small intestine and colon were opened lengthwise, embedded in paraffin, sectioned (6 μm), and stained with hematoxylin-eosin (H&E) and Alcian blue-safranin O (pH 0.4). The sections were analyzed with a light microscope (Nikon, Tokyo, Japan).
Mucosal specimens of ileum and colon sections were fixed with 3% glutaraldehyde in 0.1 mol/L cacodylate buffer (pH 7.2) for 2 h at 4 °C. These samples were then washed several times with the same buffer and postfixed with osmium tetroxide for 2 h at 4 °C. Specimens were washed with 0.1% sodium acetate, stained en bloc with 2% uranyl acetate, dehydrated through ethanol, and embedded in Spurr抯 low-viscosity resin. Representative areas were sectioned and stained with toluidine blue. The selected fields were trimmed further for ultrathin section and stained with 3% uranyl acetate in 3% ethanol, followed by treatment with Reynolds lead citrate. Ultrathin sections were examined under a Hitachi H-600 transmission electron microscope.
The H&E stained sections were coded and evaluated at 40× and 100× by an unbiased observer. Mucosal damage was classified as follows[7]: 0 (normal), 1 (surface epithelium damaged), 2 (<50% mucosa damaged), 3 (>50% mucosa damaged) and 4 (entire mucosa damaged). In each section, multiple readings of mucosal damage were obtained at 500 μm intervals. The mean of these 15-20 readings for each section provided a mucosal damage index for that section.
Mucosal injury was quantitated under light microscope. Specimens were embedded in paraffin, section was cut and stained with H&E. Measurements of villous height in microns (measured from the villous tip to the muscularis mucosa) were made in the control and experimental groups with an objective micrometer. Average value was calculated and documented. All the calculation was done being unaware of the details by an experienced pathologist.
Goblet cells were identified by their classical morphology in each small intestine and colon section in all rats. Total goblet cell number was determined along the combined crypt-villi axis of 10 well-oriented crypt-villus units in each small bowel section and 10 well-oriented colonic crypt units, from the crypt base to the luminal surface[8]. To confirm goblet cell numbers identified by morphology, goblet cells in small intestine and colon sections from the same animals were localized using Alcian blue staining. Positive staining with Alcian blue indicates the presence of acid mucins within goblet cells and was quantitated as outlined for morphological assessment.
Urinary L and M concentrations and the L/M ratio were determined using GC-9A high performance gas chromatography with pulsed electrochemical detector. Increased intestinal permeability was defined by a L/M ratio >0.03. Markedly increased intestinal permeability was defined by a L/M ratio >0.10.
The LPS concentration in portal venous blood was determined using Limulus test kit II (Medical Science Research Institute, Shanghai); 0.05 mL of Limulus reagent was added to 0.1 mL of plasma pretreated with modified perchloric acid and mixed frequently, then it was water bathed for 25 min at 37 °C. The solution was added with Limulus tripeptide 0.05 mL again and water bathed for 3 min at 37 °C, then sodium nitrite, sulfamoyl ammonium and naphthyl ethylidene, each of which was 0.5 mL, were added one by one, and mixed frequently. Using 722- Spectrophotometer (Optical Instrument Station, Shanghai), an A-value of absorbance was obtained after shade selection, then the LPS concentration (EU/mL) was obtained from the standard curve.
All comparisons were made by analysis of variance with multiple comparison tests. All data were expressed as the mean±SD. Statistical significance was considered to be reached, when P value was 0.05 or less. The linear trends were measured by linear correlation coefficients and corresponding tests.
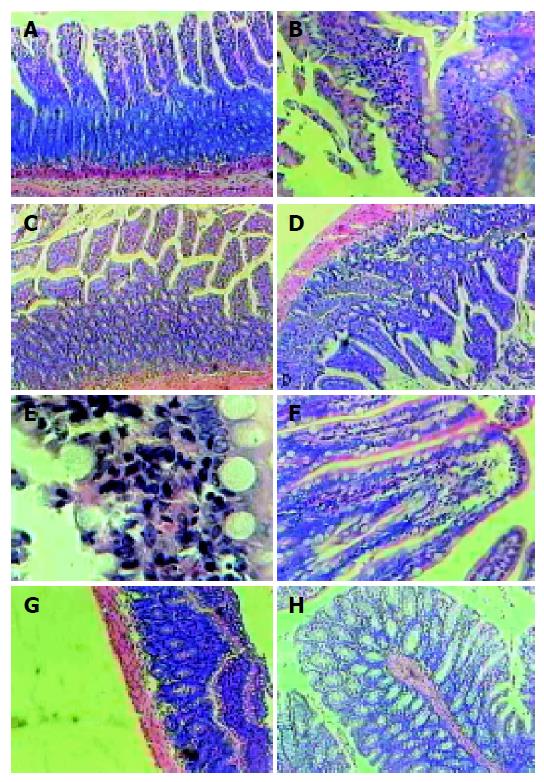
The control specimens showed normal and typical leaf-like villi crypts. In contrast, the specimens from the experimental group after shock resuscitation showed different degrees of structural changes ranging from swelling and degeneration of villous epithelial cells to extensive denudation and collapse of the villi. The severity of the damage was clearly related to the time of resuscitation after ischemic shock. At the beginning after restoration of blood pressure, an increased degree of mucosal injury was observed. The major injury occurred at the 3rd h after shock resuscitation of the small intestine, which was heavily injured. Following tissue restitution, the denuded mucosal surface covered intensively by goblet cells was found at the 3rd h. But the injury was still serious and heavy inflammatory cell infiltration occurred at this time. Mucosal restitution was reestablished in most of the injured area after 6 h, totally completed at 12-24 h. Compared to the small intestine, the injury to the large intestine was less severe (Figure 1).
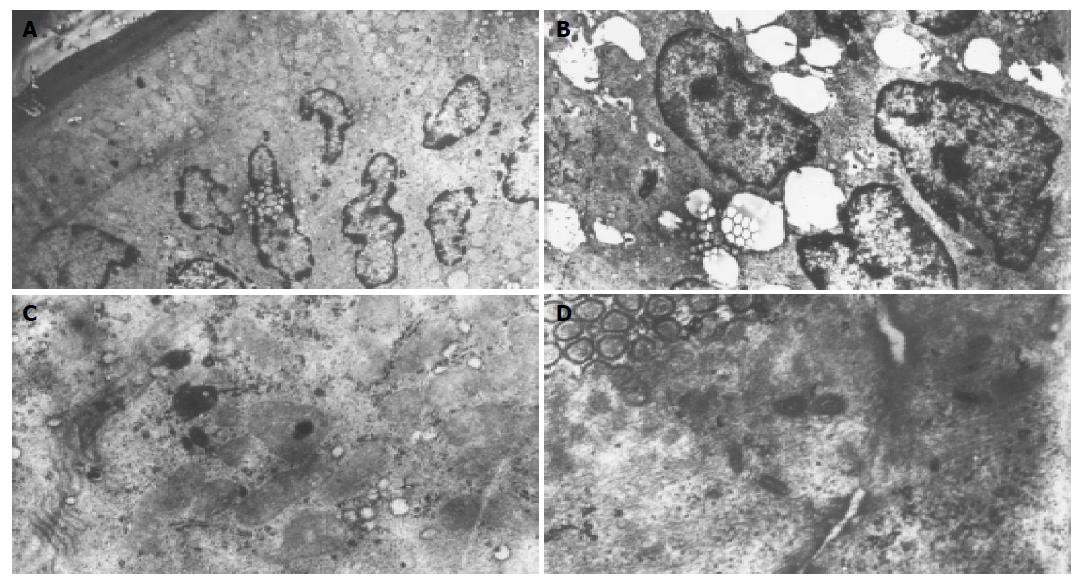
Electron microscopic examination of the intestinal mucosa revealed some changes as follows (Figure 2): the apoptosis and necrosis were major injury mode in the intestinal mucosa. There were a lot of enterocytes with the chromatins compacting and segregating against the nuclear envelope between normal appearing cells, and membranes enclosed with cytoplasmic blebs were observed. Some detached cells had characteristic features of apoptosis, but still preserved microvilli and intact mitochondria. The others showed the swelling of mitochondria and endoplasmic reticulum, scarce and lodging microvilli and opening of tight junction among the cells. No apoptotic features were found in control intestinal epithelium except at the tips of the villi.
In the small intestine, experimental groups showed a progressive reduction of the mucosal thickness (jejunum and ileum: r = -0.898 and -0.829 respectively, all P<0.001) and the villous height (jejunum and ileum: r = -0.758 and -0.659 respectively, all P<0.001). The average height of villi significantly decreased from 309±24 µm in the control to 231±28 µm in the 24 h group in jejunum, from 271±31 µm in the control to 204±23 µm in the 24 h group in ileum. As compared to the control group, the mucosal thickness at 24 h diminished by 25.2%, 23.6%, 23.4% and 24.5% respectively, namely from 547±23 µm to 418±28 µm in jejunum, 483±45µm to 364±35 µm in ileum. The mucosal thickness of colon was not markedly changed in each time point group (F = 0.296, P = 0.936, Table 1).
| Villous height (μm) | Mucosal thickness (μm) | |||||
| Group | Jejunum | Ileum | Jejunum | Ileum | Colon | |
| Con | 310±30 | 270±22 | 545±13 | 482±19 | 633±58 | |
| Exp(h) | 0 | 309±24 | 271±31 | 547±23 | 483±45 | 651±65 |
| 1 | 297±29 | 244±25a | 523±18a | 445±26a | 639±60 | |
| 3 | 278±24a | 210±27a | 501±27a | 420±31a | 674±58 | |
| 6 | 264±19a | 228±32a | 470±31a | 394±27a | 664±71 | |
| 12 | 252±25a | 220±18a | 433±19a | 380±33a | 652±69 | |
| 24 | 231±28a | 204±23a | 418±28a | 364±35a | 631±78 | |
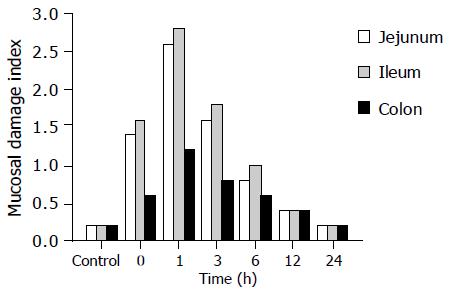
Control samples taken from small intestine and colon exhibited a minor grade of damage at each time point group. In the experimental group, the 0th h after shock resuscitation showed moderate damage with the values of the mucosal damage index being 1.4 in jejunum, 1.6 in ileum and 0.6 in colon. The value was significantly elevated in the 1st h group and the 3rd h group with their damage grade being 2.6 in jejunum, 2.8 in ileum and 1.2 in colon, then it dropped gradually at the 6th h (0.8, 1.0, and 0.6, respectively in the above three areas). In the 12th h, all mucosal damage and degradation were nearly closer to that in the control group. For the mucosal thickness change, the damage in colon was significantly less (Figure 3).
Intestinal permeability to macromolecules, as measured by the urinary concentration of L and M, was significantly increased in experimental group rats. The value of L/M was elevated following the time, and reached the peak from 3 to 6 h (0.063±0.012, 0.098±0.011 respectively, P<0.001), then decreased significantly at the 12th h, but was still higher than that in the control group (0.049±0.016, P<0.001). The value of L/M was approximate to that of the control group in 24th h group (0.037±0.011, P>0.05). The increase and decrease trends of L/M, with 6 h as the turning point, are both of statistical significance (before 6 h: r = -0.786, P<0.001; after 6 h: r = -0.767, P<0.001). In general, the change of LPS concentration from portal venous plasma was similar to that of L and M in urine (Table 2 and Figure 4).
In the experimental group, the number of goblet cells decreased gradually by 35.4% and 20.0% after shock resuscitation, from 24313 of the control group to 157±9 of the 24th h group in the ileum (r = -0.910, P<0.001), 310±19 of the control group to 24818 of 24th h group in the colon (r = 0-0.437, P<0.001). But the decrease degree in colon mucosa was less than that in the ileum (Table 3).
A major function of the gut is to prevent the absorption of toxins, antigens, protease and micro-organisms across the intestinal wall. Epithelial cells cover the surface of the gastrointestinal tract, serving as a barrier between the luminal and tissue compartments[9,10]. Hemorrhagic shock results in a condition of incomplete ischemia of the gut. The ischemia of the small intestine leads rapidly to an impairment of the protective barrier function of the mucosa, and thus the earliest restitution of the mesenteric blood flow is necessary. Although it is evident that circulatory re-establishment is vital in rescuing hypoxic tissues, it is well known that reperfusion would lead to a paradoxical injury. Ikeda et al, had reported that the characteristic feature of ischemia/reperfusion injury to the small mucosal epithelium is the detachment of enterocytes from the basement membrane and the apoptosis is regarded as major injury form[11-13]. In this study, two distinct models on cell death-apoptosis and necrosis were involved in the destruction of rat intestinal mucosal epithelium through electron microscopy.
Based on semiquantitative histological examination and the mucosal damage index, we found that the small intestine is more susceptible to ischemia/reperfusion injury than the colon (mucosal damage index: ileum vs colon in 3rd h group was 2.8 vs 1.2).These data provide probably an explanation for the clinical observation that patients with small intestinal ischemia tend to have worse outcomes than large intestine ischemia. This would also explain why there is a minor injury in the colon. Allopurinol, a competitive inhibitor of xanthine oxidase, is more effective in diminishing the exacerbation of injury in the small intestine after reperfusion because of the level of xanthine oxidase being much higher in the small intestine than that in the colon. However, the exact mechanism why the injury in colon was less severe than in the small intestine is not totally clear. Inherent anatomical structure and biological function of large intestine would be the principal factors against injury.
Repair of intestinal mucosa is called as restitution, where epithelial cells near the wound rapidly flatten, extend with lamellipodia and migrate to form an intact epithelial barrier[14]. The present study suggests that goblet cells were involved in the process of restitution of the small intestine subjected to superficial ischemia/reperfusion injury. Accumulation of goblet cells observed at villous tips by light microscopy in our study seems to be the mode of rapid mucosal repair as restitution. Masuda et al[15], reported, in the recent study, that no incorporation of 5-bromodeoxyuridine (BrdU) was found in the goblet cells covering the villus tips indicating that the accumulation of goblet cells was not due to cell proliferation[16,17].
The decreased number of goblet cells could be an explanation for the reason they participate in the process of the intestinal mucosal restitution. Goblet cells reside throughout the length of intestine and are responsible for the production and maintenance of the mucous blanket by synthesizing and secreting mucins which can protect the intestinal mucosal epithelium from damage by preventing bacteria and toxin from accessing the underlying mucosa[18-20]. Goblet cells can discharge mucins in response to reactive oxygen species, inflammatory mediators and LPS[21,22]. By means of increasing mucins production and secretion, goblet cells play a function against intestinal injury. A goblet cell has only a time of secretory course from its differentiation to death, so overexpression of mucins will result, when there is a decrease in number. The present study suggests that the mucins were upregulated at sites of gastric and intestinal mucosa injury[23]. On the other hand, intestinal mucosal atrophy would be associated with a reduction in the number of goblet cells.
Maintenance of the intestinal barrier function depends on the integrity of cellular plasma membrane, tight junctions as well as the elaboration of endothelial and epithelial secreting products[24]. Ischemia/reperfusion injury of the gut after hemorrhagic shock lead to epithelial injury and increased intestinal mucosal permeability LPS are the major outer surface membrane components containing in almost all Gram-negative bacteria and act as extremely strong stimulators of tissue damage[25]. LPS flowing across the basement membrane into the lamina propria and portal vein indicated loss of the gut barrier function, while it was difficult to detect LPS from portal vein clinically. The metabolically inactive tracers L and M used in this study can determine whether and when an increase of intestinal permeability occurred in severe stress and how the degree of permeability increase correlated with the severity of initial injury. In the hemorrhagic shock setting, an increase in the L/M ratio corresponding to increased intestinal permeability was observed in rats. The intestinal mucosal injury exactly induced a marked increase of intestinal permeability (L/M>0.10). At the same time, the degree of mucosal damage index and histological alteration in initial injury (in 3rd h group) correlated with the increase in intestinal permeability (including L/M ratio and concentration of LPS). But, there was no significant positive correlation between the morphometric alteration and the degree of increased intestinal permeability. The degree of intestinal mucosal injury did not appear to be consistent with the value of L/M and LPS in urine and plasma. After the restitution had been reestablished, the intestinal permeability was still increased (L/M = 0.039±0.016; LPS 0.15±0.045, P<0.05). It means that the developing of leakage of intraluminal markers was behind the morphological alteration. This also suggested that the restitution on the injury mucosa did not achieve its completely normal condition. Complete repair of injury mucosa takes several days because it concerns a process involving mitosis[26,27].
In conclusion, the gut barrier was subjected to ischemia/reperfusion injury after hemorrhagic shock. Damaged intestinal mucosal cells showed two distinct models of cell death-apoptosis and necrosis. The injury of gut barrier resulted in an increase in permeability to intraluminal substances. It is uncertain whether there is a correlation between the morphological alteration and the degree of increased intestinal permeability. The morphological alteration after intestinal mucosal injury cannot reflect the function of gut barrier. On the other hand, goblet cells appear to be intimately involved in restitution of the epithelium. The decreased number of goblet cells perhaps represent the increasing consumption of the goblet cell itself and its secreting products. Compared to the small intestine, the large intestine has stronger potentiality against injury.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Wilmore DW, Smith RJ, O'Dwyer ST, Jacobs DO, Ziegler TR, Wang XD. The gut: a central organ after surgical stress. Surgery. 1988;104:917-923. [PubMed] |
| 2. | Deitch EA, Forsythe R, Anjaria D, Livingston DH, Lu Q, Xu DZ, Redl H. The role of lymph factors in lung injury, bone marrow suppression, and endothelial cell dysfunction in a primate model of trauma-hemorrhagic shock. Shock. 2004;22:221-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 93] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 3. | Davidson MT, Deitch EA, Lu Q, Osband A, Feketeova E, Németh ZH, Haskó G, Xu DZ. A study of the biologic activity of trauma-hemorrhagic shock mesenteric lymph over time and the relative role of cytokines. Surgery. 2004;136:32-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Ackland G, Grocott MP, Mythen MG. Understanding gastrointestinal perfusion in critical care: so near, and yet so far. Crit Care. 2000;4:269-281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Kanwar S, Windsor AC, Welsh F, Barclay GR, Guillou PJ, Reynolds JV. Lack of correlation between failure of gut barrier function and septic complications after major upper gastrointestinal surgery. Ann Surg. 2000;231:88-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Chaudry IH. Rat and mouse models of hypovolemic-traumatic shock. Pathophysioloty of shock, sepsis and organ failure. Berlin: Springer Verlag 1993; 371. [DOI] [Full Text] |
| 7. | Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg. 1970;101:478-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1258] [Cited by in RCA: 1427] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 8. | Fernandez-Estivariz C, Gu LH, Gu L, Jonas CR, Wallace TM, Pascal RR, Devaney KL, Farrell CL, Jones DP, Podolsky DK. Trefoil peptide expression and goblet cell number in rat intestine: effects of KGF and fasting-refeeding. Am J Physiol Regul Integr Comp Physiol. 2003;284:R564-R573. [PubMed] |
| 9. | Baumgart DC, Dignass AU. Intestinal barrier function. Curr Opin Clin Nutr Metab Care. 2002;5:685-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 173] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 10. | Gewirtz AT, Liu Y, Sitaraman SV, Madara JL. Intestinal epithelial pathobiology: past, present and future. Best Pract Res Clin Gastroenterol. 2002;16:851-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Ikeda H, Suzuki Y, Suzuki M, Koike M, Tamura J, Tong J, Nomura M, Itoh G. Apoptosis is a major mode of cell death caused by ischaemia and ischaemia/reperfusion injury to the rat intestinal epithelium. Gut. 1998;42:530-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 235] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 12. | Zhang C, Sheng ZY, Hu S, Gao JC, Yu S, Liu Y. The influence of apoptosis of mucosal epithelial cells on intestinal barrier integrity after scald in rats. Burns. 2002;28:731-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Noda T, Iwakiri R, Fujimoto K, Matsuo S, Aw TY. Programmed cell death induced by ischemia-reperfusion in rat intestinal mucosa. Am J Physiol. 1998;274:G270-G276. [PubMed] |
| 14. | Ikeda H, Yang CL, Tong J, Nishimaki H, Masuda K, Takeo T, Kasai K, Itoh G. Rat small intestinal goblet cell kinetics in the process of restitution of surface epithelium subjected to ischemia-reperfusion injury. Dig Dis Sci. 2002;47:590-601. [RCA] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Masuda K, Ikeda H, Kasai K, Fukuzawa Y, Nishimaki H, Takeo T, Itoh G. Diversity of restitution after deoxycholic acid-induced small intestinal mucosal injury in the rat. Dig Dis Sci. 2003;48:2108-2115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Itoh H, Yagi M, Fushida S, Tani T, Hashimoto T, Shimizu K, Miwa K. Activation of immediate early gene, c-fos, and c-jun in the rat small intestine after ischemia/reperfusion. Transplantation. 2000;69:598-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Itoh H, Yagi M, Hasebe K, Fushida S, Tani T, Hashimoto T, Shimizu K, Miwa K. Regeneration of small intestinal mucosa after acute ischemia-reperfusion injury. Dig Dis Sci. 2002;47:2704-2710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Kim KC, McCracken K, Lee BC, Shin CY, Jo MJ, Lee CJ, Ko KH. Airway goblet cell mucin: its structure and regulation of secretion. Eur Respir J. 1997;10:2644-2649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Belley A, Keller K, Göttke M, Chadee K. Intestinal mucins in colonization and host defense against pathogens. Am J Trop Med Hyg. 1999;60:10-15. [PubMed] |
| 20. | Smirnova MG, Guo L, Birchall JP, Pearson JP. LPS up-regulates mucin and cytokine mRNA expression and stimulates mucin and cytokine secretion in goblet cells. Cell Immunol. 2003;221:42-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 150] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 21. | Verburg M, Renes IB, Van Nispen DJ, Ferdinandusse S, Jorritsma M, Buller HA, Einerhand AW, Dekker J. Specific responses in rat small intestinal epithelial mRNA expression and protein levels during chemotherapeutic damage and regeneration. J Histochem Cytochem. 2002;50:1525-1536. [RCA] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Blanchard C, Durual S, Estienne M, Bouzakri K, Heim MH, Blin N, Cuber JC. IL-4 and IL-13 up-regulate intestinal trefoil factor expression: requirement for STAT6 and de novo protein synthesis. J Immunol. 2004;172:3775-3783. [PubMed] |
| 23. | Itoh H, Beck PL, Inoue N, Xavier R, Podolsky DK. A paradoxical reduction in susceptibility to colonic injury upon targeted transgenic ablation of goblet cells. J Clin Invest. 1999;104:1539-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Nusrat A, Turner JR, Madara JL. Molecular physiology and pathophysiology of tight junctions. IV. Regulation of tight junctions by extracellular stimuli: nutrients, cytokines, and immune cells. Am J Physiol Gastrointest Liver Physiol. 2000;279:G851-G857. [PubMed] |
| 25. | Alexander C, Rietschel ET. Bacterial lipopolysaccharides and innate immunity. J Endotoxin Res. 2001;7:167-202. [PubMed] |
| 26. | Renes IB, Verburg M, Bulsing NP, Ferdinandusse S, Büller HA, Dekker J, Einerhand AW. Protection of the Peyer's patch-associated crypt and villus epithelium against methotrexate-induced damage is based on its distinct regulation of proliferation. J Pathol. 2002;198:60-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Prasad M, Ito S, Silen W. Functional studies of in vitro rat distal colon before and after restitution. Surgery. 1997;121:430-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |