Published online Sep 21, 2005. doi: 10.3748/wjg.v11.i35.5460
Revised: April 6, 2005
Accepted: April 9, 2005
Published online: September 21, 2005
AIM: To study the distributions and frequencies of intestinal endocrine cells in the C57BL/6 mouse with immunohistochemical method using seven types of specific antisera against chromogranin A (CGA), serotonin, somatostatin, glucagons, gastrin, cholecystokinin (CCK)-8 and human pancreatic polypeptide (hPP) after abdominal subcutaneous implantation of murine lung carcinoma (3LL).
METHODS: The experimental animals were divided into two groups, one is non-implanted Sham and the other is 3LL-implanted group. Samples were collected from six regions of intestinal tract at 28th d after implantation of 3LL cells (1×105 cell/mouse).
RESULTS: In this study, five types of immunoreactive (IR) cells were identified except for gastrin and hPP. The regional distributions of the intestinal endocrine cells in the 3LL-implanted group were similar to those of the non-implanted Sham. However, significant decreases of IR cells were detected in 3LL-implanted group compared to those of non-implanted Sham. CGA- and serotonin-IR cells significantly decreased in 3LL-implanted groups compared to that of non-implanted Sham. Somatostatin-IR cells in the jejunum and ileum and CCK-8-IR cells in the jejunum of 3LL-implanted groups significantly decreased compared to that of non-implanted Sham. In addition, glucagon-IR cells were restricted to the ileum and colon of non-implanted Sham.
CONCLUSION: Implantation of tumor cell mass (3LL) induced severe quantifiable changes of intestinal endocrine cell density and the abnormality in density of intestinal endocrine cells may contribute to the development of gastrointestinal symptoms such as anorexia and indigestion, frequently encountered in patients with cancer.
- Citation: Ku SK, Seong SK, Kim DY, Lee HS, Kim JD, Choi HY, Seo BI, Lee JH. Changes of the intestinal endocrine cells in the C57BL/6 mouse after implantation of murine lung carcinoma (3LL): An immunohistochemical quantitative study. World J Gastroenterol 2005; 11(35): 5460-5467
- URL: https://www.wjgnet.com/1007-9327/full/v11/i35/5460.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i35.5460
A system using the 3-Lewis lung carcinoma (3LL), a tumor which once arose spontaneously in C57BL/6 mice, is particularly useful because it metastasizes into the lungs. Now, 3LL is considered as one of the widely used type of tumor in the development and study of antitumoral agents[1,2]. C57BL/6 mouse is an inbred black mouse and is probably the most widely used of all inbred strains, though in many ways it seems to be atypical of inbred strains of laboratory mice. This strain of mice is resistant to chloroform toxicity[3], to induction of cleft palate by cortisone, to lethal effects of ozone[4] and to colon carcinogenesis by 1,2-dimethylhydrazine[5]. In addition, it is also a recommended host for the following transplantable tumors: 3LL, mammary adenocarcinoma BW 10232, melanoma B16, myeloid leukemia C 1498 and preputial gland carcinoma ESR586 T.
Gastrointestinal endocrine cells dispersed in the epithelia and gastric glands of the digestive tract synthesize various kinds of gastrointestinal hormones and play an important role in the physiological functions of the alimentary tract. Until now, the investigation of gastrointestinal endocrine cells is considered to be an important part of a phylogenic study[6] and the endocrine cells are regarded as the anatomical units responsible for the production of gut hormones, and the change in their density would reflect a change in the capacity of producing these hormones[7]. Many studies have elucidated the regional distribution and relative frequency of different endocrine cells in the gastrointestinal tract (GIT) of the various vertebrates and also the researches or data processing about gastrointestinal endocrine cells in the mouse strains have been widely executed including normal C57BL/6 mouse[8]. The changes of gastrointestinal endocrine cells in some diseases are also well demonstrated. An abnormal density of gastrointestinal endocrine cells was reported in patients with diabetes[7] and proliferation of endocrine cells in the epithelium was detected in pernicious anemia[9]. Some quantitative changes of endocrine cells were also demonstrated in celiac sprue[10] and in pancreatectomy[11]. In addition, changes of endocrine cells in colorectal carcinoma induced by chemicals were demonstrated[12].
In cancer patients, the most frequent and distressing symptoms are gastrointestinal disorder, and Komurcu et al[13], reported that dry mouth, weight loss, early satiety, taste changes, constipation, anorexia, bloating, nausea, abdominal pain and vomiting were 10 most common gastrointestinal symptoms in patients with lung, breast and prostate cancer. In the change of the gastrointestinal endocrine cells with tumor, most of the reports were related to the induction of gastrointestinal endocrine tumor by some chemicals[14,15] or by some diseases[16]. In addition, the composition, number, and types of endocrine cells in the endocrine tumors located in the gastrointestinal tract are well recognized[17-19].
Although nearly one-half of the most frequently reported and most distressing symptoms in patients with cancer are gastrointestinal in nature[13], the study about changes of gastrointestinal endocrine cells was restricted to the region of endocrine carcinoid tissues or nonneoplastic mucosa around the carcinoids. In addition, there was no report dealing with changes of gastrointestinal endocrine cell profiles after subcutaneous implantation of tumor. The objective of this study was to clarify the changes of the endocrine cells in the intestinal regions of C57BL/6 mouse after subcutaneous implantation of 3LL by specific immunohistochemistry using seven types of antisera against chromogranin A (CGA), serotonin, somatostatin, glucagons, gastrin, cholecystokinin (CCK)-8 and human pancreatic polypeptide (hPP).
Twenty adult female C57BL/6 mice (6-wk old, 21-26 g of body weight upon receipt) were acquired from the Charles River Laboratories (Yokohama, Japan) and used in this study after acclimatization for 1 wk. Animals were allocated five per autoclaved filter-top cages (Nalgene, Rochester, NY, USA) in a temperature (20-25 °C) and humidity (50-55%) controlled room during acclimatization periods. Light:dark cycle was 12:12 h and sterilized feed (Samyang, Korea) and autoclaved water were supplied, with free access. Animals were divided into two groups, 3LL-implanted group and non-implanted Sham group. Each of 10 mice was used in this study. All procedures in this study were in compliance with the Animal Welfare Act Regulations (9CFR Parts 1, 2 and 3) and with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996).
Lewis lung carcinoma was maintained as subcutaneous tumor mass. Maintained subcutaneous tumor mass was excised under sterile conditions and single cell suspensions were prepared by collagenase type IV and DNase I (Sigma, USA) in PBS and filtration of the resulting tumor cell suspension through cell strainer (Costar, USA). After counting and adjusting the cell number (1×105 cell/mouse), 3LL cell was subcutaneously implanted at abdominal skin with viable tumor cells.
After 28 d of implantation, all experimental animals were anesthetized with diethyl ether. For inducing gastric and/or intestinal emptying, animals were fasted for about 24 h. After phlebotomization, samples from six regions of the intestinal tract, the duodenum, jejunum, ileum, cecum, colon and rectum were fixed in Bouin’s solution.
After paraffin embedding, 3-4 μm serial sections were prepared. Representative sections of each tissue were stained with hematoxylin and eosin for light microscopic examination of the normal gastrointestinal architecture.
Each representative section was deparaffinized, rehydrated and immunostained with the peroxidase anti-peroxidase (PAP) method[20]. Blocking of nonspecific reaction was performed with normal goat serum, prior to incubation with the specific antisera (Table 1). After rinsing with phosphate buffered saline (PBS; 0.01 mol/L, pH 7.4), the sections were incubated in secondary antiserum. They were then washed in PBS buffer and finally the PAP complex was prepared. The peroxidase reaction was carried out in a 3, 3’-diaminobenzidine tetrahydrochloride solution containing 0.01% H2O2 in Tris-HCl buffer (0.05 mol/L, pH 7.6). After immunostaining, the sections were lightly counterstained with Mayer’s hematoxylin and the immunoreactive (IR) cells were observed under light microscope.
| Antisera raised1 | Code | Source | Dilution |
| CGA2 | A430 | DAKO Corp., Carpinteria, CA, USA | 1:1 000 |
| Serotonin | BO68082C | BioGenex Lab., San Ramon, CA, USA | 1:20 |
| Somatostatin | PUO421295 | BioGenex Lab., San Ramon, CA, USA | 1:20 |
| Glucagon | 927604 | Dia Sorin, Stillwater, MN, USA | 1:2 000 |
| Gastrin | PUO190796 | BioGenex Lab., San Ramon, CA, USA | 1:20 |
| CCK-82 | 750257 | Dia Sorin, Stillwater, MN, USA | 1:500 |
| HPP2 | A610 | DAKO Corp., Carpinteria, CA, USA | 1:600 |
The specificity of each immunohistochemical reaction was determined as recommended by Sternberger[20], including the replacement of specific antiserum by the same antiserum, which had been preincubated with its corresponding antigen.
In the restricted view fields on a computer monitor using automated image analysis process (Soft Image System, Germany) attached to light microscopy, IR cells showing immunoreactivities against each antiserum were counted among 1 000 epithelial and intestinal acinar cells. The frequencies of IR cells were calculated as mean±SD of total 10 parts (one filed in each animal) of each intestinal region.
Mann-Whitney U-Wilcoxon rank sum W test (M-W test) was used to analyze the significance of data with SPSS for Windows (Release 6.1.3, SPSS Inc., USA) and the significant values were represented by a and b (P<0.05; P<0.01).
In this study, five kinds of the IR endocrine cells were detected against CGA, serotonin, somatostatin and CCK-8 in the intestine of C57BL/6 mice regardless of implantation (Tables 2 and 3). However, glucagon-IR cells were restricted to non-implanted Sham and no gastrin- and hPP-IR cells were demonstrated in this study. According to the location of the intestines, different regional distributions and frequencies of these IR cells were observed and these differences are shown in Table 2. Most of these IR cells in the epithelial regions were generally spherical or spindle in shape, while occasionally round in shape cells were also found in the intestinal gland regions. In addition, most of these IR cells showed significant (P<0.01 or P<0.05) decrease in 3LL-implantation group compared to that of non-implanted Sham group.
| Antisera | Non-implanted Sham | 3LL-implanted group | ||||
| Duodenum | Jejunum | Ileum | Duodenum | Jejunum | Ileum | |
| CGA1 | 24.70±4.11 | 21.10±3.70 | 9.30±1.95 | 13.20±3.79b | 7.20±3.12b | 2.10±1.29b |
| Serotonin | 22.80±4.76 | 12.20±4.24 | 6.10±1.73 | 11.10±4.20b | 9.10±1.60a | 4.10±0.99b |
| Somatostatin | 1.70±3.01 | 6.10±1.91 | 2.60±0.84 | 1.60±0.84 | 0.90±0.57b | 1.40±0.70b |
| Glucagon | ND2 | ND | 1.40±0.52 | ND | ND | ND |
| CCK-81 | 8.30±2.71 | 3.40±1.35 | ND | 9.40±2.55 | 1.30±0.48b | ND |
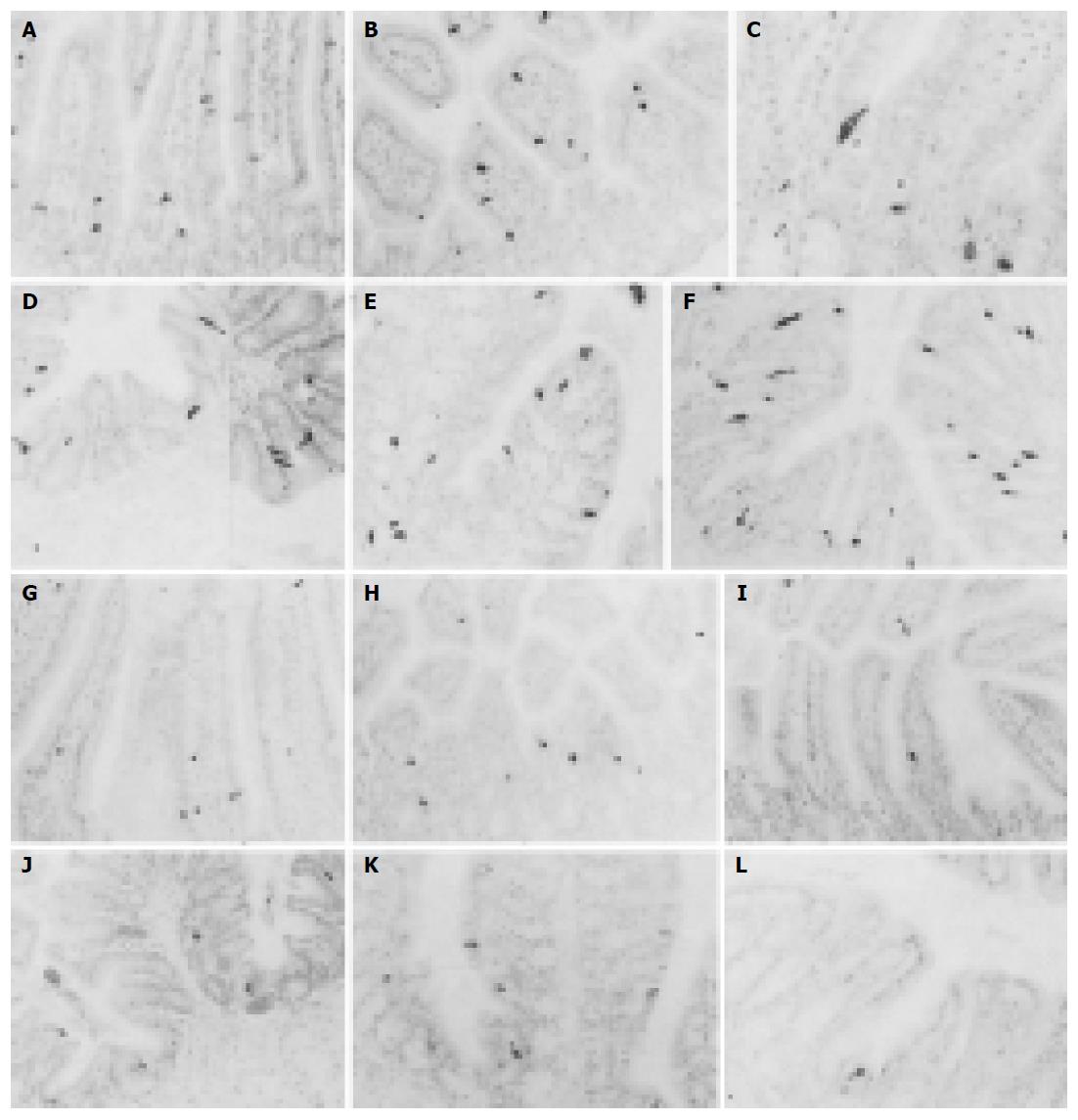
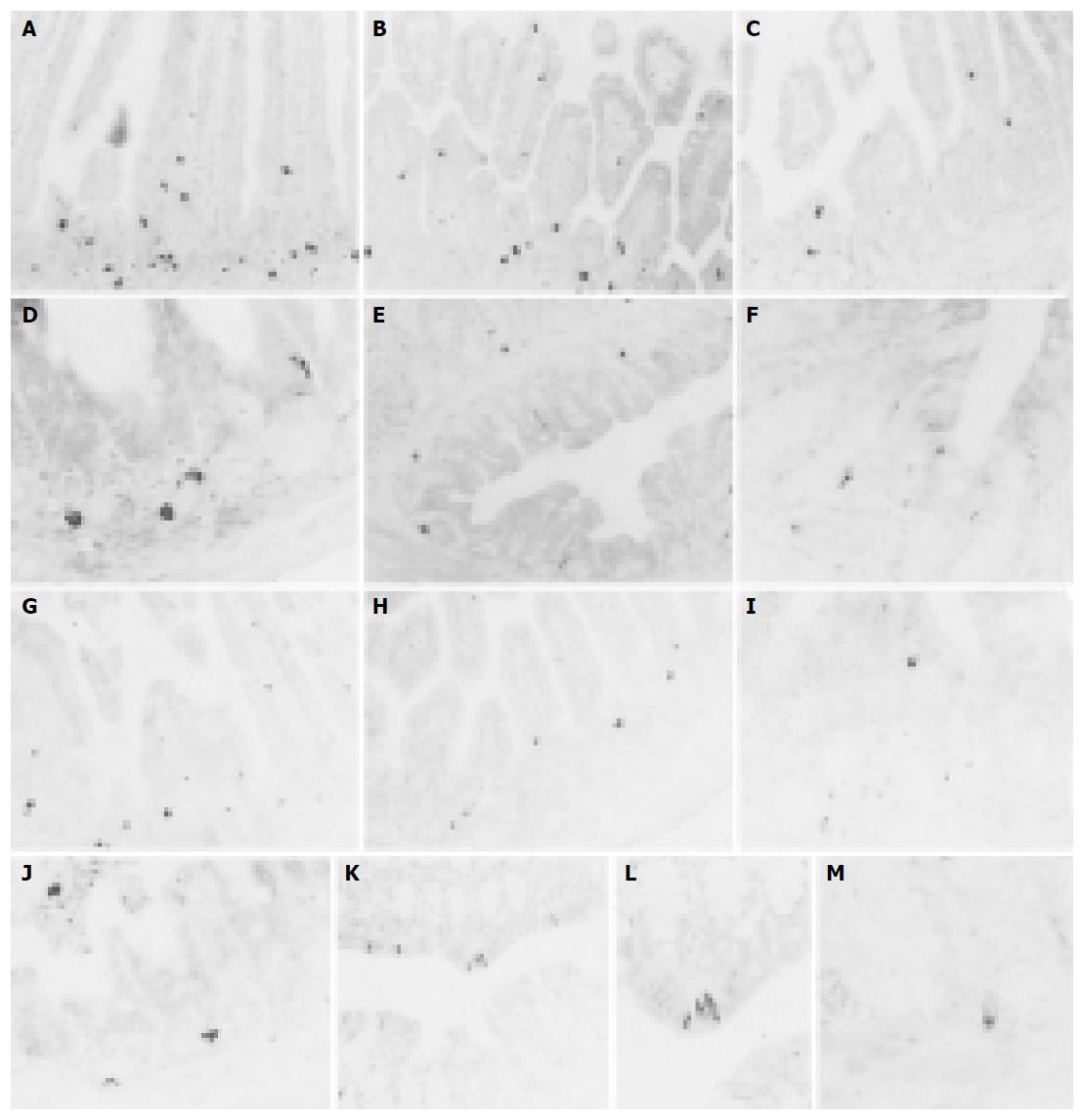
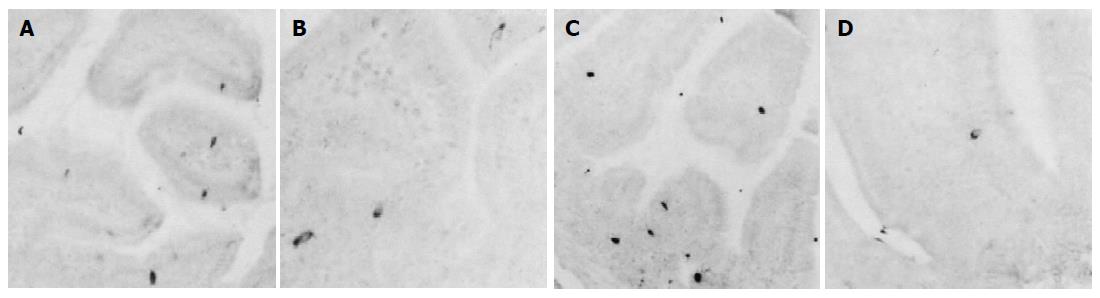
CGA-IR cells were observed in the entire intestinal regions of both groups. They were mainly dispersed in the basal portions of intestinal mucosal regions rather than the surface epithelial regions and they were more numerously detected in the small intestine compared to that of the large intestine regardless of implantation of 3LL except for the colon in which CGA-IR cells were more numerously detected than that of the jejunum and ileum (Figures 1A-M). In addition, CGA-IR cells in the colon of 3LL-implanted group that were restricted to the surface epithelium differed from that of non-implanted Sham (Figures 1E, K and L). The frequency of CGA-IR cells in the entire intestinal regions of 3LL-implanted group significantly (P<0.01) decreased compared to that of non-implanted Sham (Tables 2 and 3). About 46.56%, 65.88%, 77.42%, 69.86%, 40.14% and 75.00% of CGA-IR cells in the duodenum, jejunum, ileum, cecum, colon and rectum of 3LL-implanted group decreased compared to that of non-implanted Sham, respectively.
Serotonin-IR cells were observed in the entire intestinal regions of both groups. These IR cells were dispersed throughout whole gastric mucosa, mainly in the surface epithelium and the intestinal gland regardless of 3LL-implantation (Figures 2A-L). They showed the highest frequency in the rectum of non-implanted Sham and in the colon and duodenum of 3LL-implanted group. The frequency of serotonin-IR cells in the entire intestinal regions of 3LL-implanted group was significantly (P<0.01 or P<0.05) decreased compared to that of non-implanted Sham (Tables 2 and 3). About 51.32%, 25.41%, 32.79%, 42.28%, 59.86% and 95.24% of serotonin-IR cells in the duodenum, jejunum, ileum, cecum, colon and rectum of 3LL-implanted group were decreased compared to that of non-implanted Sham, respectively.
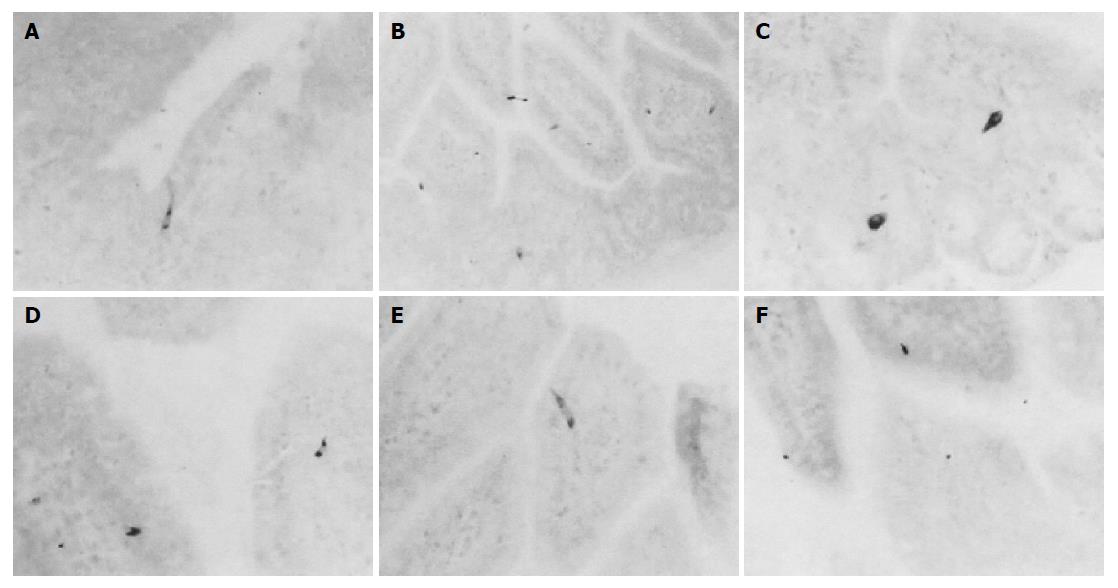
Somatostatin-IR cells were restricted to the small intestine regions of non-implanted Sham and 3LL-implanted groups. These IR cells were dispersed in the surface epithelium and intestinal gland regions of the mucosa of both groups (Figures 3A-F). The frequency of somatostatin-IR cells in the duodenum of 3LL-implanted group was similar to that of non-implanted Sham (about 5.88% was decreased). However, cells in the jejunum and ileum of 3LL-implanted group significantly (P<0.01) decreased compared to that of non-implanted Sham (about 85.25% and 46.15% were decreased, respectively, Tables 2 and 3).
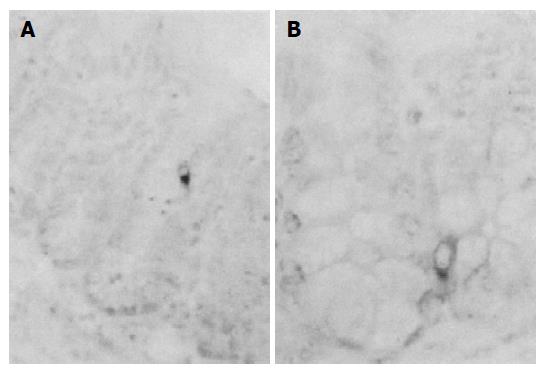
A few glucagon-IR cells were restricted to the intestinal gland regions of ileum and colon of non-implanted Sham (Figures 4A and B). However, no glucagon-IR cells were observed in 3LL-implanted group (Tables 2 and 3).
No gastrin-IR cells were demonstrated in the entire intestinal regions of non-implanted Sham and 3LL-implanted groups (Tables 2 and 3).
CCK-8-IR cells were restricted to the duodenum and jejunum of both groups and most of these IR cells were dispersed in the surface epithelium and intestinal gland of the intestinal mucosa similar to that of other IR cells (Figures 5A-D). The frequency of CCK-8-IR cells in the duodenum of 3LL-implanted group was similar to that of non-implanted Sham (about 13.25% was increased). However, cells in the jejunum of 3LL-implanted group were significantly (P<0.01) decreased compared to that of non-implanted Sham (about 61.76% were decreased, Tables 2 and 3).
No hPP-IR cells were demonstrated in this study, regardless of implantation of 3LL (Table 2).
In the present study, the changes of the endocrine cells in the intestinal regions of C57BL/6 mouse after subcutaneous implantation of 3LL were observed by specific immuno-histochemistry. As results of 3LL implantation, most of IR cells were significantly (P<0.01 or P<0.05) decreased in the entire intestinal tract except for somatostatin- and CCK-8-IR in the duodenum in which similar cell numbers were detected between non-implanted Sham and 3LL-implanted groups. In the rectum, the most dramatical changes were demonstrated and the most dramatical changes were demonstrated in serotonin-IR cells. These changes might be inducing a gastrointestinal disorder observed in patients with cancer[13].
Chromogranins have been found to occur in a large variety of endocrine organs and cells outside the adrenal medulla, and they have been claimed as common “markers” of all neuroendocrine cells[21]. Although, seldom there were changes of frequency of CGA-IR cells in the GIT of Rodentia, Qian et al[22], reported that decrease of CG-IR cells was demonstrated in the intestine of unilateral cervical vagotomized mouse. In addition, increase of CG-IR cells was also demonstrated in the intestine of proton pump inhibitor treated rat[23] and celiac children[24]. According to the previous reports, it is considered that changes of CG-IR cells were possible as a sign of disease and these changes were also considered as results of changes of other endocrine cells because CGs were regarded as a common marker of other endocrine cells[21]. In the present study, CGA-IR cells were remarkably decreased in the intestine of 3LL-implanted groups compared to that of non-implanted Sham.
Serotonin consisted of monoamines widely distributed in the nervous system and gastro-entero-pancreatic endocrine cells and the main functions of serotonin were inhibition of gastric acid secretion and contraction of smooth muscle in the GIT[25]. Serotonin-IR cells were dramatically decreased in the duodenum of diabetic mouse[26] and the number of these IR cells and occupied area were significantly decreased in the damaged gastroduodenal regions of mouse, induced by ethanol[27]. In the present study, among used antisera serotonin-IR cells were most significantly decreased in the entire regions of the intestine. These changes might induce gastrointestinal problems, especially some clinical signs related to gastric motility and gastric acid secretion.
Somatostatin consisting of 14 amino acids was isolated from hypothalamus of sheep for the first time and it could be divided into straight form and cyclic form[28]. This substance inhibits the secretion of the other neuroendocrine hormones[29]. It is known that somatostatin-IR cells show the widest distribution in the whole GIT except for the large intestine of all vertebrate species investigated, including the primitive agnathans with serotonin-IR cells[8]. Somatostatin-IR cells were significantly increased in the cancer adjacent mucosa compared to that of cancer distant mucosa of colorectal endocrine cancer patients[30] and decrease of these IR cells was demonstrated in the duodenal ulcer patients with Helicobacter pylori, but they were increased to normal after eradication of H pylori[31]. In the present study, somatostatin-IR cells were remarkably decreased in the jejunum and ileum of 3LL-implanted groups compared to that of non-implanted Sham. This decrease of somatostatin-IR cells is considered to have induced somewhat serious problems to gastrointestinal physiology, particularly their functions on the digestive tract.
Glucagon is synthesized in the A cells of the pancreas and regulates serum glucose levels. These IR cells have been demonstrated in various mammals. However, there were no reports dealing with the evidence of changes of glucagon-IR cells in any disease case or in any abnormality induced by any chemical or any manipulation. In the present study, corresponding to that of previous study[8], they were restricted to the ileum and colon of non-implanted Sham. However, no glucagon-IR cells were demonstrated in the intestine of 3LL-implanted group.
Gastrin secreted by intestinal G cell, promotes gastric acid secretion and CCK secreted by intestinal I cell stimulates the pancreatic enzyme secretion. However, gastrin-IR cells in the C57BL/6 mice were restricted to the stomach regions[8] and they were not detected in this study. Enteric CCK-8-IR cells significantly increased in celiac sprue[11]. In the present study, marked decrease of CCK-8-IR cells were demonstrated in the jejunum, after implantation of 3LL and these results induce some gastric disorders. Since PP was isolated from insulin extraction of pancreas at 1961, the regional distribution of PP-IR cells in the mouse strains was relatively well known, but strain-dependant differences existed among mice[8,32]. In the C57BL/6 mice, generally no hPP-IR cells were demonstrated in the GIT[8].
In conclusion, implantation of tumor cell mass (3LL) induce severe quantifiable changes of the intestinal endocrine cell density and the abnormality in density of endocrine cells may contribute to the development of gastrointestinal symptoms such as anorexia and indigestion, frequently encountered in patients with cancer. However, the overall hormone states such as amounts in blood in 3LL-implanted animals were not observed in this study, the exact meaning of the decrease of endocrine cells is unclear. Therefore, to understand the exact relationship between the decrease of GI endocrine cells and gastrointestinal disorder observed in patients with cancer, some quantified study of blood hormone states must be made.
Co-first-authors: Sae-Kwang Ku and Hyeung-Sik Lee
Science Editor Guo SY Language Editor Elsevier HK
| 1. | DeWys WD. A quantitative model for the study of the growth and treatment of a tumor and its metastases with correlation between proliferative state and sensitivity to cyclophosphamide. Cancer Res. 1972;32:367-373. [PubMed] |
| 2. | Moon EY, Seong SK, Jung SH, Lee M, Lee DK, Rhee DK, Pyo S, Yoon SJ. Antitumor activity of 4-phenyl-1-arylsulfonylimidazolidinone, DW2143. Cancer Lett. 1999;140:177-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Deringer MK, Dunn TB, Heston WE. Results of exposure of strain C3H mice to chloroform. Proc Soc Exp Biol Med. 1953;83:474-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Goldstein BD, Lai LY, Ross SR, Cuzzi-Spada R. Susceptibility of inbred mouse strains to ozone. Arch Environ Health. 1973;27:412-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Evans JT, Shows TB, Sproul EE, Paolini NS, Mittelman A, Hauschka TS. Genetics of colon carcinogenesis in mice treated with 1,2-dimethylhydrazine. Cancer Res. 1977;37:134-136. [PubMed] |
| 6. | D'Este L, Buffa R, Pelagi M, Siccardi AG, Renda T. Immunohistochemical localization of chromogranin A and B in the endocrine cells of the alimentary tract of the green frog, Rana esculenta. Cell Tissue Res. 1994;277:341-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | El-Salhy M, Sitohy B. Abnormal gastrointestinal endocrine cells in patients with diabetes type 1: relationship to gastric emptying and myoelectrical activity. Scand J Gastroenterol. 2001;36:1162-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Ku SK, Lee HS, Lee JH. An immunohistochemical study of the gastrointestinal endocrine cells in the C57BL/6 mice. Anat Histol Embryol. 2003;32:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Rode J, Dhillon AP, Papadaki L, Stockbrügger R, Thompson RJ, Moss E, Cotton PB. Pernicious anaemia and mucosal endocrine cell proliferation of the non-antral stomach. Gut. 1986;27:789-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Buchan AM, Grant S, Brown JC, Freeman HJ. A quantitative study of enteric endocrine cells in celiac sprue. J Pediatr Gastroenterol Nutr. 1984;3:665-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Ravazzola M, Baetens D, Engerman R, Kovacevic N, Vranic M, Orci L. Endocrine cells in oxyntic mucosa of a dog 5 years after pancreatectomy. Horm Metab Res. 1977;9:480-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Sitohy B, El-Salhy M. Colonic endocrine cells in rats with chemically induced colon carcinoma. Histol Histopathol. 2001;16:833-838. [PubMed] |
| 13. | Komurcu S, Nelson KA, Walsh D, Ford RB, Rybicki LA. Gastrointestinal symptoms among inpatients with advanced cancer. Am J Hosp Palliat Care. 2002;19:351-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Wängberg B, Nilsson O, Theodorsson E, Modlin IM, Dahlström A, Ahlman H. Are enterochromaffinlike cell tumours reversible? An experimental study on gastric carcinoids induced in Mastomys by histamine2-receptor blockade. Regul Pept. 1995;56:19-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Solcia E, Fiocca R, Villani L, Luinetti O, Capella C. Hyperplastic, dysplastic, and neoplastic enterochromaffin-like-cell proliferations of the gastric mucosa. Classification and histogenesis. Am J Surg Pathol. 1995;19 Suppl 1:S1-S7. [PubMed] |
| 16. | Sjöblom SM, Sipponen P, Karonen SL, Järvinen HJ. Mucosal argyrophil endocrine cells in pernicious anaemia and upper gastrointestinal carcinoid tumours. J Clin Pathol. 1989;42:371-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Iwafuchi M, Watanabe H, Ishihara N, Shimoda T, Iwashita A, Ito S. Peptide YY immunoreactive cells in gastrointestinal carcinoids: immunohistochemical and ultrastructural studies of 60 tumors. Hum Pathol. 1986;17:291-296. [RCA] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Yasui W, Sumiyoshi H, Hata J, Mandai K, Tahara E. Gut endocrine cells in rat stomach carcinoma induced by N-methyl-N'-nitro-N-nitrosoguanidine. J Cancer Res Clin Oncol. 1986;111:87-92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Shimamoto F, Tahara E, Yanaihara N. Gut endocrine cells in rat intestinal-tract carcinoma induced by 1,2-dimethylhydrazine. J Cancer Res Clin Oncol. 1983;105:221-230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Sternberger LA. The unlabeled antibody peroxidase-antiperoxidase (PAP) method. Immunocytochemistry. New York: John Wiley Sons 1979; 104-169. |
| 21. | Cohn DV, Elting JJ, Frick M, Elde R. Selective localization of the parathyroid secretory protein-I/adrenal medulla chromogranin A protein family in a wide variety of endocrine cells of the rat. Endocrinology. 1984;114:1963-1974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 142] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Qian BF, el-Salhy M, Danielsson A, Shalaby A, Axelsson H. Effects of unilateral cervical vagotomy on antral endocrine cells in mouse. Histol Histopathol. 1999;14:705-709. [PubMed] |
| 23. | White SL, Smith WC, Fisher LF, Gatlin CL, Hanasono GK, Jordan WH. Quantitation of glandular gastric changes in rats given a proton pump inhibitor for 3 months with emphasis on sampling scheme selection. Toxicol Pathol. 1998;26:403-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Mariani P, Pietroletti R, D'Alessandro MD, Castro M, Lucidi V, Ceccamea A, Lomanto D, Carlei F, Lezoche E. [Changes in the prostanoid content and the population of endocrine cells in jejunal biopsies from celiac children]. Minerva Pediatr. 1990;42:131-134. [PubMed] |
| 25. | Guyton AC. Secretory functions of the alimentary tract. Textbook of medical physiology. Philadelphia: WB Saunders 1988; 801-815. |
| 26. | Spångéus A, Forsgren S, El-Salhy M. Effect of diabetic state on co-localization of substance P and serotonin in the gut in animal models. Histol Histopathol. 2001;16:393-398. [PubMed] |
| 27. | Penissi A, Mariani L, Souto M, Guzmán J, Piezzi R. Changes in gastroduodenal 5-hydroxytryptamine-containing cells induced by dehydroleucodine. Cells Tissues Organs. 2000;166:259-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 28. | Brazeau P, Vale W, Burgus R, Ling N, Butcher M, Rivier J, Guillemin R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973;179:77-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2618] [Cited by in RCA: 2363] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 29. | Kitamura N, Yamada J, Calingasan NY, Yamashita T. Immunocytochemical distribution of endocrine cells in the gastrointestinal tract of the horse. Equine Vet J. 1984;16:103-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Zhao R, Wang Y, Chen T. Somatostatin and its secretory cells in tumor surrounding mucosa in colorectal cancer patients and its significance. Zhonghua WaiKe ZaZhi. 1997;35:268-270. [PubMed] |
| 31. | Queiroz DM, Mendes EN, Rocha GA, Moura SB, Resende LM, Barbosa AJ, Coelho LG, Passos MC, Castro LP, Oliveira CA. Effect of Helicobacter pylori eradication on antral gastrin- and somatostatin-immunoreactive cell density and gastrin and somatostatin concentrations. Scand J Gastroenterol. 1993;28:858-864. [RCA] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 76] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Ku SK, Lee JH, Lee HS, Park KD. The regional distribution and relative frequency of gastrointestinal endocrine cells in SHK-1 hairless mice: an immunohistochemical study. Anat Histol Embryol. 2002;31:78-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |