Published online Sep 14, 2005. doi: 10.3748/wjg.v11.i34.5332
Revised: January 23, 2005
Accepted: January 26, 2005
Published online: September 14, 2005
AIM: To report the comprehensive diagnosis and treatment of acute rejection in the first case of living-related small bowel transplantation with a long-term survival in China.
METHODS: A 18-year-old boy with short gut syndrome underwent living-related small bowel transplantation, with the graft taken from his father (44-year old). A segment of 150-cm distal small bowel was resected from the donor. The ileo-colic artery and vein from the donor were anastomosed to the infrarenal aorta and vena cava of the recipient respectively. The intestinal continuity was restored with an end-to-end anastomosis between the recipient jejunum and donor ileum, and the distal end was fistulized. FK506, MMF and prednisone were initially used for post-transplant immunosuppression. Endoscopic observation and mucosal biopsies of the graft were carried out through the terminal ileum enterostomy; serum was collected to detect the levels of IL-2R, IL-4, IL-6 and IL-8. The change of the graft secretion and absorption was observed.
RESULTS: Acute rejection was diagnosed promptly and cured. The patient was in good health, 5 years after living-related small bowel transplantation.
CONCLUSION: The correct diagnosis and treatment of acute rejection are the key to the long-term survival after living-related small bowel transplantation.
- Citation: Song WL, Wang WZ, Wu GS, Li MB, Li JP, Ji G, Dond GL, Zhang HW. Diagnosis and treatment of acute rejection in the first case of human living-related small bowel transplantation with a long-term survival in China. World J Gastroenterol 2005; 11(34): 5332-5335
- URL: https://www.wjgnet.com/1007-9327/full/v11/i34/5332.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i34.5332
The first patient after small bowel transplantation in China has survived for more than 5 years. The prompt diagnosis and treatment of acute rejection following transplantation are essential for a successful transplantation and long survival. However, there is no conventional standard for acute rejection diagnosis following small bowel transplantation. The present paper describes its comprehensive diagnosis post-transplantation and control.
The patient, a 18-year-old man, suffered from volvulus necrosis and underwent a partial enterectomy in September 1998, the remaining jejunum was only 40 cm long. He was diagnosed as having short bowel syndrome. He was 185 cm high and weighed only 35 kg. He received intravenous feeding for a rather long period of time following transplantation. On May 20, 1999, he underwent living-related small bowel transplantation with a 150 cm long terminal ileum from his father as the donor intestine. The superior mesenteric artery from the donor was anastomosed to the infrarenal aorta and the mesenteric vein from the donor superior was anastomosed to the recipient portal vein or inferior vena cava. The arteries and veins from the donor intestine were anastomosed to the recipient’s abdominal aorta and inferior vena cava in an end-to-side fashion respectively. The recipient’s intestine was cut at 10 cm prior to the ileum. The proximal end of intestine from the donor was anastomosed to the proximal end of the recipient’s jejunum in an end-to-end fashion. The distal end of intestine from the donor was anastomosed to the distal end of the recipient’s jejunum in a side-to-end fashion. An intestinal fistula was made at 10 cm prior to graft as a viewing fenestra[1-4]. The recipient was given anticoagulant and anti-infectious treatment and total parenteral nutrition. He was treated with FK506, MMF and hydroprednisone and their combinations. On the next day following transplantation, intravenous administration of FK506 was replaced by intra-intestinal administration. The patient recovered after transplantation. He could take fluid food 2 d after transplantation. On d 31 after transplantation, the absorption rate of xylitol was 30.6%. A month after transplantation he could feed himself, and gained weight steadily. MMF was stopped and the dosage of hydroprednisone was reduced. Discharge increased at the site of the intestinal fistula, and reached more than 1 500 mL/d, 60 d after transplantation, which suggested acute rejection. On the basis of the graft absorptive functional changes in the immunological indexes, endoscopic and pathologic examination, he was diagnosed as acute rejection and administered an impact treatment with a large dosage of immunosuppressive agents. Three days later, the symptoms were alleviated and all the indexes returned to normal gradually. Acute rejection did not occur again. One year after transplantation, the intestinal fistula was closed. At present, the patient is still alive, weighing 56-58 kg[5-8].
Olympus GIF-XQ230 electron gastroscope was used to observe the mucous membrane with the remaining jejunum as a control. Mucous membrane from different sites was taken for pathological and microbiological examination. When we closed the intestinal fistula, we could examine the graft through the anus. However, no abnormal symptom has occurred since then.
Substrate coloration with ABTS was carried out, standard curve was drawn according to the standard, and then the serum levels of SIL-2R, IL-6, and IL-8 were determined with antagonistic sandwich ELISA kit. The serum level of IL-4 was determined with the biosource cytoscreen solid-phase sandwich ELISA kit (USA) for IL-4 detection. The above-mentioned four indexes were re-examined every 3 mo.
D-xylitol absorption test was done to detect the absorptive function of the graft. The discharge at the site of the graft fistula and its changes were observed. The stool was also observed after the fistula was closed. The test was done every 3 mo.
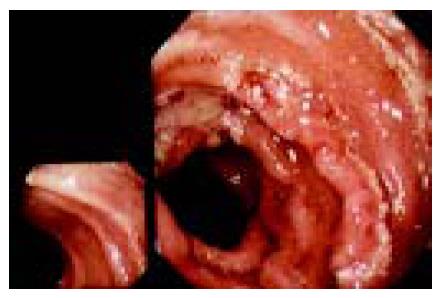
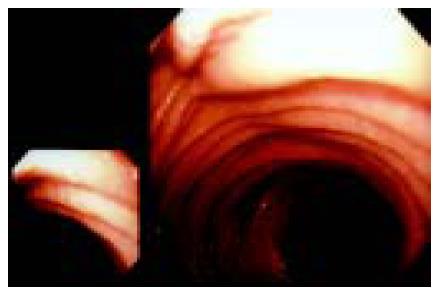
The graft membrane was present with the edema. The membrane appeared as an intensified light reflection. Yellowish white mucus was attached to the graft membrane and there were hemorrhage, erosions and round ulcers (0.3-0.6 cm in diameter) and linear ulcers (Figures 1 and 2). Under endoscope, the graft membrane was encrusted with yellowish white thin mucus. The graft wall was brittle and apt to bleed when touched, and it bled a lot when biopsy specimen was taken. The graft canal had hypanakinesia. On the other hand, these symptoms were absent in the remaining jejunum. Erosion occurred where there were lesions, some epithelial cells appeared in an atrophic short column shape, goblet cells became smaller or even disappeared. There was a general edema in lamina propria. Neutrophilic granulocytes were present at the lamina propria and tunica muscularis. Infiltration occurred in plasma cells and lymphocyte, neutrophilic granulocytes in blood vessels increased. Microbiological examination was negative and pathological examination showed that the patient’s remaining jejunum was almost normal. When the intestinal fistula was closed one year later, the patient had no abnormal symptoms.
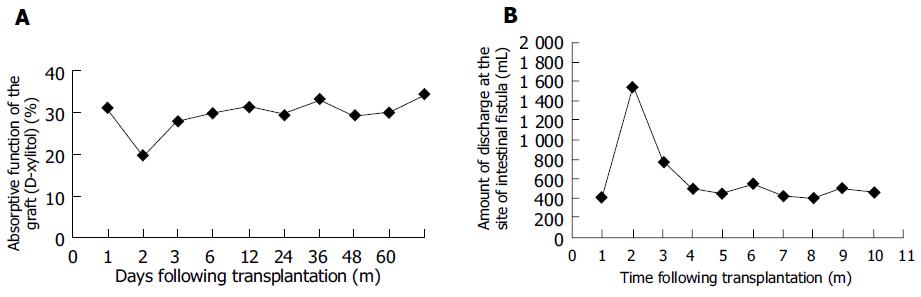
The absorptive function of the graft returned to normal 30 d after transplantation (the absorption rate of xylitol was 30.6%) and remained normal until the onset of rejection. Sixty-five days after transplantation, the absorption rate dropped to 24.6% and on 67th d, 19.6% increased gradually after treatment and returned to normal again 80 d after transplantation (Figure 3A). The discharge at the intestinal fistula remained normal just after transplantation. Sixty days after transplantation, the discharge began to increase and reached 1 520 mL, 61 d after transplantation. The discharge decreased after treatment and returned to normal, 80 d after transplantation (Figure 3B). Then, the absorptive function of the graft was examined every 3 mo and no change was found. After the intestinal fistula was closed, the patient’s stool was solid and no loose and watery stool occurred.
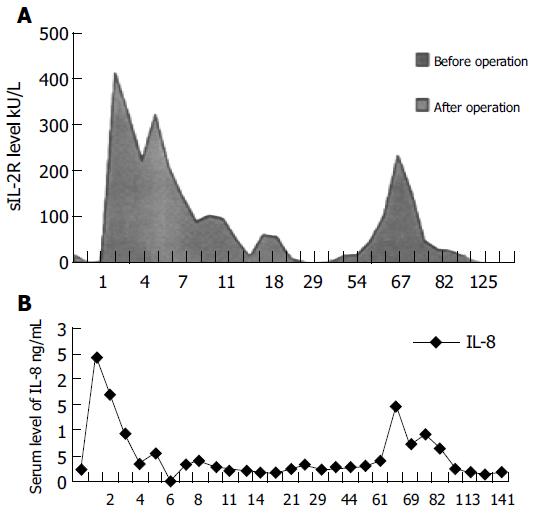
The serum level of SIL-2R was 15.68 kU/L before transplantation, reached its first peak one day after transplantation, began to increase again 65 d after transplantation and reached its second peak 67 d after transplantation (243.98 kU/L). After treatment, its level decreased gradually and returned to normal, 80 d after transplantation (Figure 4A). The serum level of IL-8 was 0.26 µg/L before transplantation, reached its first peak one day after transplantation, began to increase again 65 d after transplantation, and reached its second peak 67 d after transplantation (1.47 µg/L). The level of IL-8 began to drop slowly when rejection was treated, and returned to the level before transplantation, 96 d after transplantation (Figure 4B). The serum level of IL-4 and IL-6 had no change before and after transplantation. Then, the four indexes were examined every 3 mo and no change was found.
Small bowel transplantation is the only therapy for end-stage intestinal diseases. However, since the small bowel is characterized by the existence of plenty of bacteria and a high immunogenicity, it is prone to rejection, which usually results in a transplantation failure. The incidence rate of acute rejection after small bowel transplantation is as high as 90% and acute rejection usually occurs 4-60 d after transplantation[9]. Prompt diagnosis and treatment of acute rejection after transplantation are crucial to a successful small bowel transplantation. However, the detecting indexes, which are instrumental in suggesting acute rejection of the graft, have not been identified yet. Based on the clinical symptoms, endoscopic and pathological findings and changes in absorptive function of the graft, acute rejection was diagnosed 67 d after transplantation. Following the control of immunological indexes and absorptive function of the graft, no significant changes were found and acute rejection did not recur.
The first endoscopic examination was done 15 h following transplantation, once every day in the first 3 d, once every 2-3 d after 3 d, and once or twice every month after month. When there was evidence for acute rejection, endoscopic examination was performed more frequently. Since acute rejection of the graft is heterogeneous, biopsy specimens were taken at more than one site from the graft with the remaining jejunum as a control. Microbiological examination was done to exclude the risk for other intestinal pathological changes. The patient had an increased intestinal discharge and his immunological indexes were changed 2 mo after transplantation. Endoscopic examination and pathologic biopsy specimens suggested acute rejection, which accords with the findings of previous studies[9-12]. Treatment with intensive immunosuppressive drugs alleviated the symptoms. Endoscopic and pathological examinations are now essential for the control of acute rejection following transplantation[11]. When the intestinal fistula was closed one year later, no abnormal symptom was found.
The presence of congestion, edema, sporadic ulcer and necrosis on the graft at the early stage of acute rejection after transplantation, is theoretically followed by increased discharge and decreased absorptive function of the graft. However, it has not been reported in any clinical case report. The present study showed that at the early stage of acute rejection after transplantation, increased discharge of the graft was the only clinical symptom. The absorptive rate of the graft decreased greatly and reached its lowest point when severe acute rejection occurred. After being treated with intensive immunosuppressive drugs, it returned to normal and then remained normal (30%) and no acute rejection occurred after the intestinal fistula was closed, suggesting that changes in the graft discharge and absorptive function are worth to consider in observing and identifying the presence of acute rejection of the graft.
The immunological indexes, which are instrumental in suggesting acute rejection of the graft, are still being studied. Rejection following homotransplantation is initiated by the T cell’s recognition of the graft and antigen complex, which is compatible with the patient’s own tissue with its specific T-cell antigen recognition receptor. Since the direct recognition pathway of CD4+ T cells plays an essential role in the above process and in the presence of the first acute rejection[13], and CD4+ T cells secrete such cell factors as IL-2, with which IL-2R can be combined and inhibit its mutual action with cells, the serum IL-2R level might be a sensitive immunological index of the presence of rejection following homotransplantations[13,14]. We detected many immunological indexes, which might be connected with rejection, and found that IL-2R increased one day after transplantation and then decreased gradually, but increased again and reached its second peak until acute rejection occurred, suggesting that there might be a close relationship between the serum level of IL-2R and the onset of acute rejection of the graft. Thus, the serum level of IL-2R can be used as an useful index for the onset of acute rejection following small bowel transplantation. When the graft rejection occurs, mononuclear leukocytes/macrophages, and T lymphocytes may be present with characteristic cellular infiltration, and these cells can secrete IL-8. It has been reported that the serum level of IL-8 increases in patients with acute rejection after transplantation of the liver or kidney and then decreases after being treated with intensive immunosuppressive drugs[15-17]. In the present study, the serum level of IL-8 reached its first peak one day after transplantation, which might be attributed to the long period of cold ischemia and lesions due to reperfusion. Since the serum level of IL-8 reached its second peak 67 d after transplantation, when acute rejection occurred no changes were observed after treatment with immunosuppressive drugs, there may be a close connection between the second peak of serum IL-8 and the onset of acute rejection. Therefore, it can be used as an important index for the diagnosis of acute rejection.
In addition, effective anti-infectious treatment and scientific nutrition arrangement may contribute to the survival rate of patients after the small bowel transplantation.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Wang WZ, Wu GS, Song WL, Ling R, Zhang SH, Du JJ, Chen DL, Zhao JX, Li MB, Li JP. The case of the clinical living-related small bowel transplantation. Zhonghua Waike Zazhi. 2000;38:236-238. |
| 2. | Chen DL, Wang WZ, Li MB, Wu GS. The irrigation and pre-serve of the living-Related small bowel transplantation. Zhonghua Putong Waike Zazhi. 2000;15:504-506. |
| 3. | Wang WZ, Song WL, Ling R, Wu GS, Wang G, Zhu QS, Xue YJ, Chao ZN. The management of graft blood vessel in the living-related small bowel transplantation. Zhonghua Putong Waike Zazhi. 2000;15:491-494. |
| 4. | Wang WZ, Ling R, Song WL, Zhang SH, Chen DL, Li MB, Ji G. Anagement of intestinal graft in living-related small bowel transplatation. Disi Junyi Daxue Xuebao. 2000;21:773-775. |
| 5. | Wang WZ, Song WL, Wu GS, Ling R, Pu JS, Zhang SH, Du JJ, Chen DL, Zhao JX, Xue YJ. The perioperative management of living-related small bowel transplantation. Zhonghua Putong Waike Zazhi. 2000;15:411-413. |
| 6. | Song WL, Wang WZ, Ling R, Wu GS, Zhao JX, Ji G. The prevention of the infection in the living related small bowel transplantation. Disi Junyi Daxue Xuebao. 2000;21:2-10. |
| 7. | Dong GL, Wang WZ, Wu GS, Song WL, Ji G, Luo L, Xu JL, Zhao CH. Strategy of nutritional support for a patient with partial live small bowel transplantation during perioperation. Shijie Huaren Xiaohua Zazhi. 2000;8:539-541. |
| 8. | Liu XN, Wang WZ, Song WL, Wu GS. The diagnose and treatment of the virus in the small bowel transplantation. Disi Junyi Daxue Xuebao. 2001;22:343. |
| 9. | Abu-Elmagd KM, Tzakis A, Todo S, Reyes J, Fung J, Nakamura K, Wright H, Furukawa H, Demetris J, Van Thiel DH. Monitoring and treatment of intestinal allograft rejection in humans. Transplant Proc. 1993;25:1202-1203. [PubMed] |
| 10. | Lee RG, Nakamura K, Tsamandas AC, Abu-Elmagd K, Furukawa H, Hutson WR, Reyes J, Tabasco-Minguillan JS, Todo S, Demetris AJ. Pathology of human intestinal transplantation. Gastroenterology. 1996;110:1820-1834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 191] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 11. | Su ZX, Yu LX, Huang JF. Small bowel transplantation. Moder Transplantation. 1sted. Beijing: People's Health Publishing House 1998; 616-652. |
| 12. | Hassanein T, Schade R, Soldevilla-Pico C, Tabasco-Minguillan J, Abu-Elmagd K, Furukawa H, Kadry Z, Demetris A, Tzakis A, Todo S. Clinical and endoscopic features of rejection in small bowel transplant recipients. Transplant Proc. 1994;26:1413. [PubMed] |
| 13. | Shirwan H, Leamer M, Wang HK, Makowka L, Cramer DV. Peptides derived from alpha-helices of allogeneic class I major histocompatibility complex antigens are potent inducers of CD4+ and CD8+ T cell and B cell responses after cardiac allograft rejection. Transplantation. 1995;59:401-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Shirwan H, Barwari L, Cramer DV. Rejection of cardiac allografts by T cells expressing a restricted repertoire of T-cell receptor V beta genes. Immunology. 1997;90:572-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Yagihashi A, Zou XM, Hirata K, Asanuma K, Tsuruma T, Matsuno T, Yamaguchi H, Yamashiro K, Koide S, Torimoto K. Evaluation of serum IL-8 concentrations after orthotopic liver transplantation in rats. Transplant Proc. 1995;27:1632-1633. [PubMed] |
| 16. | Boratyńska M. [Monitoring of interleukin-8 in urine and in serum of patients after kidney transplantation]. Przegl Lek. 1998;55:576-580. [PubMed] |
| 17. | Tilg H, Ceska M, Vogel W, Herold M, Margreiter R, Huber C. Interleukin-8 serum concentrations after liver transplantation. Transplantation. 1992;53:800-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |