Published online Sep 14, 2005. doi: 10.3748/wjg.v11.i34.5309
Revised: February 13, 2005
Accepted: February 18, 2005
Published online: September 14, 2005
AIM: To evaluate the cellular synthetic ability of cytokines involved in pro- and anti-inflammatory reactions in patients with AP.
METHODS: Sixty-seven patients with AP (16 severe, 51 mild) and 10 controls were included in the study. Cultures of whole blood were performed in samples obtained within the first 72 h from the onset of pain. Serum levels of interleukins (IL) 6, 8, 10, and TNF-α were measured at baseline and in the supernatant of cultures with (functional reserve) or without stimulation with phytohemaglutinin.
RESULTS: Basal levels of cytokines were significantly higher in patients with severe AP. A significant increase of all pro-inflammatory cytokines vs basal levels was observed in the supernatant after 24 h of whole blood cultures in patients, but not in controls. In contrast, IL-10 increased significantly in the supernatant of cultures only in patients with mild AP. Cells showed a statistically significant functional reserve for all IL in patients with mild, but only for pro-inflammatory cytokines in patients with severe AP.
CONCLUSION: A marked activation of immune system may be observed in patients with AP, being balanced between pro- and anti-inflammatory cytokines in patients with mild but not severe AP. A reduced functional reserve for the synthesis of IL-10 may be observed in patients with severe AP, which might lead to a worst prognosis.
- Citation: Laveda R, Martínez J, Muñoz C, Penalva JC, Sáez J, Belda G, Navarro S, Feu F, Mas A, Palazón JM, Sánchez-Payá J, Such J, Pérez-Mateo M. Different profile of cytokine synthesis according to the severity of acute pancreatitis. World J Gastroenterol 2005; 11(34): 5309-5313
- URL: https://www.wjgnet.com/1007-9327/full/v11/i34/5309.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i34.5309
Patients with acute pancreatitis (AP) show an exacerbated local inflammatory reaction, likely as the consequence of the pancreatic cellular injury[1,2], which may be followed by a generalization of the process, leading to a potentially severe systemic form of the disease with multiple organ failure and eventually death[3]. This clinical situation is at least partially mediated by a marked host’s immune response and a prolonged or exacerbated synthesis of pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α) and interleukins (IL) 1, 6 and 8[4-8]. In fact, recent findings have shown the existence of a significant correlation between the serum levels of the mentioned cytokines and the severity of AP[8-12].
The liberation of pro-inflammatory cytokines is followed by the synthesis of anti-inflammatory cytokines, such as IL-10, whose function is to counterbalance the hypothetical deleterious effects of an exacerbated immune response[7,13]. There is scarce and conflicting information regarding the possible effect of IL-10 in the control of the inflammatory process in patients with AP[14,15].
A main drawback in the study of cytokines in any clinical setting is its intermittent pattern of secretion and short half-life[16]. To overcome these complications, several approaches have been considered, such as the measurement of the more durable soluble cytokine receptors[9], or the measurement of the cytokine secretion patterns in vitro either by the culture of mononuclear cells[16] or whole blood with or without the addition of cellular stimulants.
The in vitro model has been utilized in different clinical settings[17,19], and it has revealed as simple, reproducible, and accurate method for evaluation of the cytokine secretion patterns, allowing the estimation of functional immune cellular reserve[20,21]. To our knowledge, no study has evaluated the usefulness of this method to investigate the inflammatory response in patients with AP. Then, the aims of this study have been to investigate the in vitro secretion pattern of pro- and anti-inflammatory cytokines by whole blood cultures in patients with AP, and the estimation of the functional cellular reserve.
A prospective series of patients with clinical diagnosis of AP and less than 48 h from the onset of symptoms admitted to the Gastroenterology Departments of two academic hospitals were consecutively included in a prospective study. The diagnosis of AP was defined as the presence of clinically compatible abdominal pain with an increase of serum amylase three times above the upper normal limit, evidence of pancreatic swelling by imaging techniques and exclusion of other possible causes of abdominal pain.
Patients with severe or mild forms of AP according to Atlanta’s criteria[22] of severity were analyzed separately. A group of healthy subjects were considered as controls. The Hospital General Universitario Ethics Committee approved the study protocol, and all patients gave their informed consent for the inclusion in the study.
Blood was obtained for routine hematological, biochemical, and coagulation studies within 72 h from the onset of symptoms. Ten milliliters of blood was inoculated into two rubber-sealed pyrogen-free tubes (Endo Tube ET, Chromogenix AB, Vienna, Austria). One of the aliquots was centrifuged 10 min at 3 500 r/min and stored at -70°C until analysis. Tubes were not exposed to free air.
Whole blood cultures Blood was distributed in a 24-well plate with or without the addition of 40 µL of phytohe-maglutinin (PHA) at a concentration of 1 µg/mL for 24 h at 37°C in a humidified atmosphere with 50 mL/L CO2. Supernatants were obtained by centrifugation at 2 500 r/min for 5 min and stored at -80°C until assay. The maximum period of time between being frozen and thawing was 3 mo, and none of the specimens was thawed and refrozen prior to analysis in the current study.
Measurement of TNF-α, IL-6, IL-8, and IL-10 Immunoenzymometric assays for the quantitative measurement of human TNF-α, IL-6, 8 and 10 in serum samples and in the supernatant of whole blood cultures were performed by handling Biosource IFN-γ EASIA Kit (Biosource Europe SA, Nivelles, Belgium), human TNF-α HS Quantikine, and IL-6, IL-8 and IL-10 Quantikine (R&D Systems, Minneapolis, MN, USA) according to manufacturer’s instructions. All samples were tested in duplicate and read at 450 and 490 nm in a ThermoMax microplate reader (Molecular Devices, Sunnyvale, CA, USA). The lower levels of sensitivity for measurement of cytokines were 1.7 pg/mL for TNF-α, 5 pg/mL for IL-6, 2 pg/mL for IL-8 and 10 pg/mL for IL-10.
We consider the presence of a status of cellular pre-activation, when the levels of the different cytokines measured in the supernatant of non-stimulated whole blood cultures were significantly higher to that measured in basal samples. We considered that cellular functional reserve exists for the synthesis and secretion of cytokines, when the levels of the different parameters measured in the supernatant of whole blood cultures plus the addition of PHA were higher to those measured in the non-stimulated cultures, and conversely, no functional reserve was considered, when the levels obtained with cellular stimulants were similar to those obtained without PHA.
All the observations are reported as median and 25th and 75th percentiles. Statistical differences were analyzed using the Mann-Whitney U test for the comparison among different groups and the Wilcoxon test for the comparisons within each group at different times. A P value < 0.05 indicated statistical significance. Analyses were performed with the SPSS Statistical package (SPSS Inc., version 11.0, Chicago, IL).
Sixty-seven patients diagnosed of AP were included in the study. AP was considered severe in 16 patients (6 male, mean age 66 ± 16 years) and mild in 51 cases (23 male, mean age 61 ± 16 years). Ten healthy subjects were studied as controls (6 male, mean age 45 ± 19 years). The etiology of AP was biliary in 37 patients (55.3%), alcoholic in 8 patients (11.9%) and others (hypertriglyceridemia, post-endoscopic cholangiopancreatography (ERCP), drugs or idiopathic) in 22 cases (32.8%). We did not find statistically significant differences in sex, age, and etiology of AP among groups. Five patients developed respiratory insufficiency (7.5%) and one progressed to acute renal failure (1.5%). Regarding local complications, five patients developed a pseudocyst (7.5%), pancreatic necrosis was observed in five cases (7.5%), and infected pancreatic necrosis in one patient (1.5%). Overall, seven patients died during admission (10.4%), all being considered as severe AP.
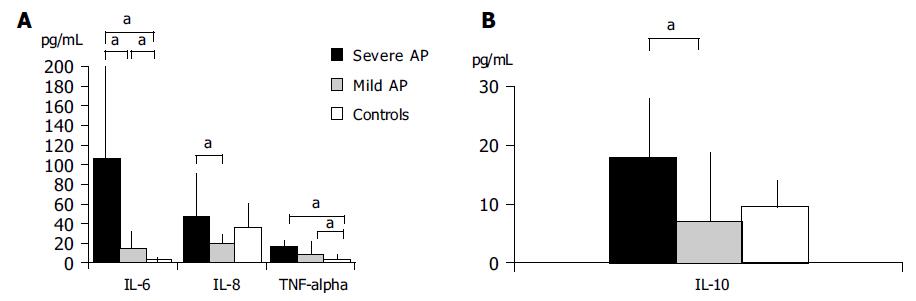
Table 1 shows the levels observed in all parameters studied in basal conditions and in the supernatant of whole blood cultures with all recorded statistical significances. Basal levels of IL-6 in patients with SAP were significantly higher than that observed in controls and patients with mild AP (P < 0.05). Basal IL-8 and TNF-α measured in patients with severe AP were significantly higher than those in patients with mild AP, but not vs healthy controls (P < 0.01 in both cases). Quite opposite, statistically significant differences were observed when comparing IL-10 levels between patients with severe vs mild AP (P < 0.05), but not vs controls (Figure 1).
| IL-6 (pg/mL) | IL-8 (pg/mL) | TNF-α (pg/mL) | IL-10 (pg/mL) | ||
| SAP | Basal | 105.6 (52.0-210)a | 46.8 (26.2-95)b | 15.8 (7.1-21.7)b | 17.7 (7.5-29.4)c |
| 24 h | 208.0 (140-476)e | 872.0 (316-3 280)e | 58.7 (52-132)e | 12.5 (8.4-34.6) | |
| 24 h+PHA | 925.0 (303-1 219)g | 2 715.0 (760-8 599)g | 473.0 (308-518)g | 7.2 (2.2-47.3) | |
| MAP | Basal | 15 (5.4-33.2) | 18.5 (1.2-33.6) | 7.9 (4.1-17.7) | 7.3 (4.1--1.6) |
| 24 h | 133.0 (53.6-856)e | 1 611.0 (484-4 336)e | 62.4 (45.5-100.5)e | 20.8 (6.5-44.1)e | |
| 24 h+PHA | 2057.0 (605-4 189)g | 8 045.0 (3 377-28 209)g | 540.5 (422-824.7)g | 51.9 (32.1-131.3)g | |
| Controls Basal | 3.2 (0.9-6.7) | 35.6 (17.9-93.7) | 4.3 (2.2-6.1) | 9.4 (6-14.4) | |
| 24 h | 7.1 (4.9-9.6) | 46.9 (32.2-94.5) | 7.1 (4.1-10.1) | 21.3 (18.3-34.5) | |
| 24 h+PHA | 610.0 (404.7-1 026)g | 1 396.0 (962-3 065.2)g | 792.0 (573.2-1 064.7)g | 53 (43.4-80.1)g | |
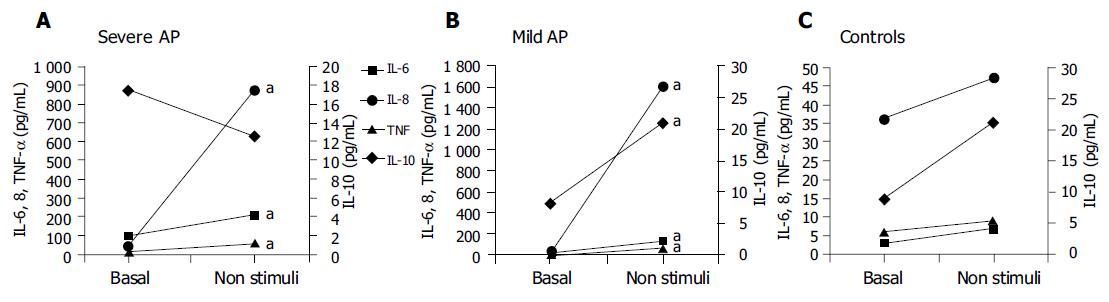
When comparing basal values with those measured in the supernatant of non-stimulated cultures, significant differences were observed in all pro-inflammatory cytokines in patients with mild or severe AP, but not in controls. IL-10 increased significantly in these experimental conditions only in patients with mild AP (Figure 2).
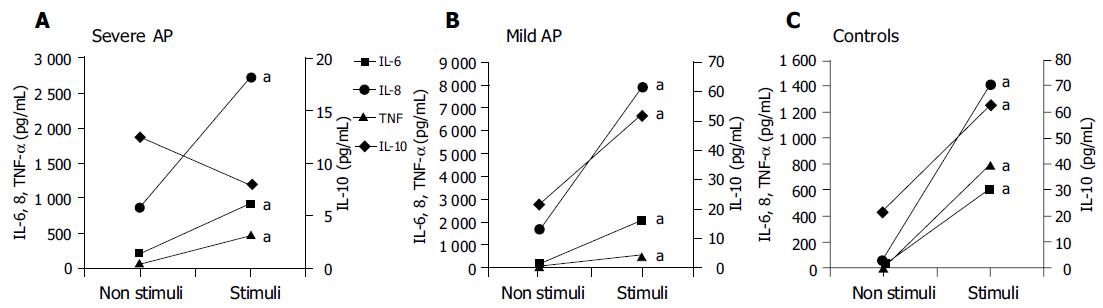
When analyzing the cellular functional reserve (i.e., values measured in the supernatant of non-stimulated vs stimulated cultures), significant increases in pro-inflammatory cytokines were observed in all groups of patients (Figure 3). However, a significant functional reserve for IL-10 was observed only in controls and in patients with mild, but not severe AP (Figure 3).
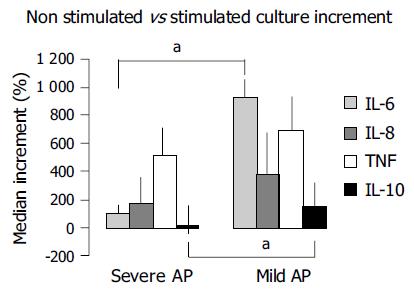
For better measurement of functional reserve, we estimated the increase observed in each studied parameter with values obtained in non-stimulated vs stimulated cultures in all three groups, and observed that this increment is higher in patients with mild than in severe AP, although this reached significance only when measuring IL-6 and IL-10 (Figure 4).
Cytokines are soluble components of the inflammatory cascade that play a seminal role in the pathogenesis of the systemic response syndrome that usually takes place in patients with AP[4-8]. Different works have shown a relationship between the levels of different pro-inflammatory cytokines reached in serum and the severity of AP, and patients with highest levels of these factors in the early phases of the disease are those developing systemic complications[8-12].
It has been recently described the anti-inflammatory role of certain cytokines, namely IL-10, and the most accepted hypothesis suggests that this is a mechanism devoted to compensate the systemic inflammatory response that precedes the development of multi-organ failure[7,13,23,24]. The existence of a misbalance between pro- and anti-inflammatory cytokines would participate in the genesis of the systemic complications that may develop in some patients with AP[25]. The information available regarding IL-10 levels in patients with AP is controversial, and the results obtained in the different studies show a great variability[13-15,23,24]. However, the experimental administration of IL-10 in animal models of induced AP has been shown to reduce cytokine release, the severity of AP and also mortality[26-28]. Similarly, the administration of this cytokine has been shown to reduce the incidence of AP in patients undergoing ERCP[29].
The discrepancies found when studying the cytokine profile in the clinical setting may be a reflection of several drawbacks inherent to the study of cytokines, such as the moment of obtaining the blood samples, its intermittent pattern of secretion, its autocrine or paracrine natural way of action[16], and short half-life. The culture of immunocompetent cells and measurement of cytokines and effector molecules in the supernatant has revealed that it is a useful method to investigate the inflammatory status and cellular functional reserve in different clinical settings[17-19]. Although early attempts with the culture of monocytes did not give satisfactory data, likely due to a different environmental situation to what is expected to find in vivo[16], the culture of whole blood and measurement of cytokines in the supernatant appears to be a simple, reproducible and accurate method in the evaluation of synthesis of components of the inflammatory cascade, since it closely approximates to what is expected to find in vivo. In addition, it allows the study not only of the basal status of cellular activation, but also of the cellular functional reserve, when comparing the spontaneous cellular ability to synthesize cytokines with that when the cells are further stimulated[20,21]. To our knowledge, this methodological approach has not been explored in patients with AP.
We have observed a spontaneous and significant increase in the synthesis of pro-inflammatory cytokines in patients, when compared to controls after 24 h of non-stimulated culture that represents the degree of cellular preactivation (Figure 2), which likely supports the contention of the existence of an inflammatory pre-activation status in patients with AP. The fact that IL-10 increases significantly in non-stimulated cultures with respect to basal values in patients with mild AP (Figure 2B) likely suggests that an anti-inflammatory response follows a parallel course to the inflammatory reaction, probably as a balancing mechanism in patients with mild AP. This situation is not found in patients with severe AP (Figure 2A), and it is similar to what is observed, when comparing the non-stimulated IL-10 synthetic ability with that after culture stimulation in this same subgroup of patients (Figure 3A), suggesting the absence of cellular functional reserve for this anti-inflammatory cytokine. The rest of the cytokines evaluated in both groups of patients show a significant increase when cultures are stimulated with PHA vs values obtained in the supernatant of cultures without stimulation (Figures 2 and 3). Then, cells obtained from patients with AP, either mild or severe, show a marked pre-activation status that is reflected in the amount of pro-inflammatory cytokines that spontaneously secrete in culture (Figure 2) and show a certain degree of cellular functional reserve when PHA is added to the culture medium (Figure 3). This is not, however, the case with respect to IL-10, as a key parameter of anti-inflammatory cytokines, since in both situations, i.e. in spontaneous culture, and after stimulation with PHA, the levels of this cytokine stabilize or even decrease (Figures 2 and 3). Figure 4 reinforces this concept when analyzing the increments of all parameters evaluated in different experimental situations.
We cannot exclude that a continuous status of inflammatory activation in patients with severe AP may lead to an early depletion of anti-inflammatory cytokines and incapacity to counterbalance the inflammatory cascade. Following this same line of thinking, it would be logical to hypothesize that an insufficient anti-inflammatory response might exert a causative effect in a subgroup of patients with AP, favoring a worse clinical evolution. The conserved cellular reserve in patients with mild AP (Figure 3B) likely supports our hypothesis.
In conclusion, the practice of whole blood cultures and measurement of cytokines either in non-stimulated vs stimulated conditions allows the estimation of the pre-activation status and the cellular functional reserve in patients with AP. In our series, the synthetic pattern of cytokines shows a different behavior in patients with mild or severe AP, and suggests that a reduced ability to synthesize anti-inflammatory cytokines in cases of severe AP may play a role in the clinical evolution of patients.
Science Editor Guo SY Language Editor Elsevier HK
Co-first-authors: Raquel Laveda and Juan Martínez
Co-correspondents: Raquel Laveda
| 1. | Weber CK, Adler G. From acinar cell damage to systemic inflammatory response: current concepts in pancreatitis. Pancreatology. 2001;1:356-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Domínguez-Muñoz JE, Viedma JA, Pérez-Mateo M, Carballo F, García Fe M. Inflammatory response in the initial phase of acute pancreatitis: relationship to the onset and severity of the disease. Rev Esp Enferm Dig. 1995;87:225-246. [PubMed] |
| 3. | de Beaux AC, Palmer KR, Carter DC. Factors influencing morbidity and mortality in acute pancreatitis; an analysis of 279 cases. Gut. 1995;37:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 185] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 4. | Bhatia M, Brady M, Shokuhi S, Christmas S, Neoptolemos JP, Slavin J. Inflammatory mediators in acute pancreatitis. J Pathol. 2000;190:117-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 5. | Kusske AM, Rongione AJ, Reber HA. Cytokines and acute pancreatitis. Gastroenterology. 1996;110:639-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 132] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 6. | Schmid RM, Adler G. Cytokines in acute pancreatitis--new pathophysiological concepts evolve. Eur J Gastroenterol Hepatol. 1999;11:125-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Schölmerich J. Interleukins in acute pancreatitis. Scand J Gastroenterol Suppl. 1996;219:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Norman J. The role of cytokines in the pathogenesis of acute pancreatitis. Am J Surg. 1998;175:76-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 508] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 9. | de Beaux AC, Goldie AS, Ross JA, Carter DC, Fearon KC. Serum concentrations of inflammatory mediators related to organ failure in patients with acute pancreatitis. Br J Surg. 1996;83:349-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 181] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 10. | Berney T, Gasche Y, Robert J, Jenny A, Mensi N, Grau G, Vermeulen B, Morel P. Serum profiles of interleukin-6, interleukin-8, and interleukin-10 in patients with severe and mild acute pancreatitis. Pancreas. 1999;18:371-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 88] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Chen CC, Wang SS, Lee FY, Chang FY, Lee SD. Proinflammatory cytokines in early assessment of the prognosis of acute pancreatitis. Am J Gastroenterol. 1999;94:213-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 123] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 12. | Hirota M, Nozawa F, Okabe A, Shibata M, Beppu T, Shimada S, Egami H, Yamaguchi Y, Ikei S, Okajima T. Relationship between plasma cytokine concentration and multiple organ failure in patients with acute pancreatitis. Pancreas. 2000;21:141-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 89] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Simovic MO, Bonham MJ, Abu-Zidan FM, Windsor JA. Anti-inflammatory cytokine response and clinical outcome in acute pancreatitis. Crit Care Med. 1999;27:2662-2665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Pezzilli R, Billi P, Miniero R, Barakat B. Serum interleukin-10 in human acute pancreatitis. Dig Dis Sci. 1997;42:1469-1472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Chen CC, Wang SS, Lu RH, Chang FY, Lee SD. Serum interleukin 10 and interleukin 11 in patients with acute pancreatitis. Gut. 1999;45:895-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | McKay CJ, Gallagher G, Brooks B, Imrie CW, Baxter JN. Increased monocyte cytokine production in association with systemic complications in acute pancreatitis. Br J Surg. 1996;83:919-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 110] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Beck J, Rondot P, Catinot L, Falcoff E, Kirchner H, Wietzerbin J. Increased production of interferon gamma and tumor necrosis factor precedes clinical manifestation in multiple sclerosis: do cytokines trigger off exacerbations? Acta Neurol Scand. 1988;78:318-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 294] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 18. | Fahey JL. Cytokines, plasma immune activation markers, and clinically relevant surrogate markers in human immunodeficiency virus infection. Clin Diagn Lab Immunol. 1998;5:597-603. [PubMed] |
| 19. | Katial RK, Hershey J, Purohit-Seth T, Belisle JT, Brennan PJ, Spencer JS, Engler RJ. Cell-mediated immune response to tuberculosis antigens: comparison of skin testing and measurement of in vitro gamma interferon production in whole-blood culture. Clin Diagn Lab Immunol. 2001;8:339-345. [PubMed] |
| 20. | De Groote D, Zangerle PF, Gevaert Y, Fassotte MF, Beguin Y, Noizat-Pirenne F, Pirenne J, Gathy R, Lopez M, Dehart I. Direct stimulation of cytokines (IL-1 beta, TNF-alpha, IL-6, IL-2, IFN-gamma and GM-CSF) in whole blood. I. Comparison with isolated PBMC stimulation. Cytokine. 1992;4:239-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 385] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 21. | van der Linden MW, Huizinga TW, Stoeken DJ, Sturk A, Westendorp RG. Determination of tumour necrosis factor-alpha and interleukin-10 production in a whole blood stimulation system: assessment of laboratory error and individual variation. J Immunol Methods. 1998;218:63-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 105] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Bradley EL. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg. 1993;128:586-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1929] [Cited by in RCA: 1734] [Article Influence: 54.2] [Reference Citation Analysis (1)] |
| 23. | Mayer J, Rau B, Gansauge F, Beger HG. Inflammatory mediators in human acute pancreatitis: clinical and pathophysiological implications. Gut. 2000;47:546-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 307] [Article Influence: 12.3] [Reference Citation Analysis (1)] |
| 24. | Brivet FG, Emilie D, Galanaud P. Pro- and anti-inflammatory cytokines during acute severe pancreatitis: an early and sustained response, although unpredictable of death. Parisian Study Group on Acute Pancreatitis. Crit Care Med. 1999;27:749-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 147] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 25. | Bone RC. Immunologic dissonance: a continuing evolution in our understanding of the systemic inflammatory response syndrome (SIRS) and the multiple organ dysfunction syndrome (MODS). Ann Intern Med. 1996;125:680-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 490] [Cited by in RCA: 457] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 26. | Rongione AJ, Kusske AM, Kwan K, Ashley SW, Reber HA, McFadden DW. Interleukin 10 reduces the severity of acute pancreatitis in rats. Gastroenterology. 1997;112:960-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 224] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 27. | Kusske AM, Rongione AJ, Ashley SW, McFadden DW, Reber HA. Interleukin-10 prevents death in lethal necrotizing pancreatitis in mice. Surgery. 1996;120:284-288; discussion 289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 135] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 28. | Van Laethem JL, Marchant A, Delvaux A, Goldman M, Robberecht P, Velu T, Devière J. Interleukin 10 prevents necrosis in murine experimental acute pancreatitis. Gastroenterology. 1995;108:1917-1922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 164] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 29. | Dumot JA, Conwell DL, Zuccaro G, Vargo JJ, Shay SS, Easley KA, Ponsky JL. A randomized, double blind study of interleukin 10 for the prevention of ERCP-induced pancreatitis. Am J Gastroenterol. 2001;96:2098-2102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 2.8] [Reference Citation Analysis (0)] |