Published online Sep 14, 2005. doi: 10.3748/wjg.v11.i34.5273
Revised: December 15, 2004
Accepted: December 21, 2004
Published online: September 14, 2005
AIM: To investigate the molecular mechanisms by which tea pigments exert preventive effects on liver carcinogenesis.
METHODS: HepG2 cells were seeded at a density of 5×105/well in six-well culture dishes and incubated overnight. The cells then were treated with various concentrations of tea pigments over 3 d, harvested by trypsinization, and counted using a hemocytometer. Flow cytometric analysis was performed by a flow cytometer after propidium iodide labeling. Bcl-2 and p21WAF1 proteins were determined by Western blotting. In addition, DNA laddering assay was performed on treated and untreated cultured HepG2 cells.
RESULTS: Tea pigments inhibited the growth of HepG2 cells in a dose-dependent manner. Flow-cytometric analysis showed that tea pigments arrested cell cycle progression at G1 phase. DNA laddering was used to investigate apoptotic cell death, and the result showed that 100 mg/L of tea pigments caused typical DNA laddering. Our study also showed that tea pigments induced upregulation of p21WAF1 protein and downregulation of Bcl-2 protein.
CONCLUSION: Tea pigments induce cell-cycle arrest and apoptosis. Tea pigments may be used as an ideal chemopreventive agent.
- Citation: Jia XD, Han C, Chen JS. Tea pigments induce cell-cycle arrest and apoptosis in HepG2 cells. World J Gastroenterol 2005; 11(34): 5273-5276
- URL: https://www.wjgnet.com/1007-9327/full/v11/i34/5273.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i34.5273
most common cause of death from cancer in the world. In general, the disease is relatively uncommon in developed countries. In the developing world, however, liver cancer is very common, accounting for more than 80% of the global cases, with China alone accounting for 55% of the worldwide incidence. Liver cancer is almost always fatal and has no effective treatment. The survival rate is low, with only a 6% 5-year survival rate in the USA. In developing countries, the survival rate is even lower. Therefore, high prevalence, high death rate, and ineffective therapy have spurred the search of novel strategies in the prevention rather than treatment of liver cancer.
Nowadays, chemoprevention is gaining more attention. This approach aims to decrease overall cancer morbidity and mortality by using substances that are capable of preventing cancer progression. Several classes of compounds have been evaluated for this purpose. Tea is one of the most popular beverages consumed in the world. The two major types of tea consumed worldwide are black tea and green tea. Green tea polyphenols and extracts have been shown in a number of animal models to prevent against chemically-induced carcinogenesis in lung, forestomach, esophagus, duodenum, pancreas, breast, and colon[1-3]. Tea pigments, a major flavonol component in black tea, have strong antioxidant potency[4]. In our previous studies, we found tea pigments to be effective in preventing the occurrence and progression of precancerous liver lesions in rats[5]. However, studies on tea pigments are still limited, especially in terms of mechanism.
The purpose of this study was to investigate the molecular mechanisms by which tea pigments exert preventive effects on liver cancer cells and to provide the scientific rationale for using tea pigments as a chemopreventive agent against liver cancer.
Tea pigments were provided by the Institute of Tea Science and Research, Chinese Academy of Agricultural Sciences (Hangzhu, China).
Human hepatoma cell line HepG2 was obtained from Beijing University Hospital (Beijing, China) and cultured in DMEM medium (Sigma, USA) supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin in a 50 mL/L CO2 atmosphere at 37°C. The cells were seeded at a density of 5×105/well in six-well culture dishes and incubated overnight. The cells then were treated with various concentrations of tea pigments over 3 d, harvested by trypsinization, and counted using a hemocytometer.
HepG2 cells were seeded at a density of 5×105/well in six-well culture dishes. To synchronize the cells at the G0 phase, HepG2 cells were incubated in serum-free medium for 24 h before treatment with tea pigments. The cells were then treated with or without 100 mg/L tea pigments and harvested at one, or after 2 and 3 d by trypsinization. The cells were centrifuged at 2 000 r/min for 5 min, washed with phosphate-buffered saline (PBS), fixed with 70% ethanol, then subjected to flow cytometric analysis on a flow cytometer after propidium iodide labeling.
Control cells, as well as cells treated with tea pigments, were harvested by scraping the cells from cultured dishes using a cell scraper and collected by centrifugation. Whole cell extracts were then prepared by lysing the cells using 4% sodium dodecyl sulfate (SDS) gel sample buffer. Cell extracts were boiled for 10 min and chilled on ice, subjected to 12% SDS-polyacrylamide gel electrophoresis, and transferred to a nitrocellulose membrane. Each membrane was cut into two pieces, one piece was incubated at 4°C overnight with p21WAF1 or Bcl-2 antibodies, and the other one with β-actin (used as a control for protein loading). All antibodies were obtained from Zymed Laboratories (USA). Then membranes were incubated at 37°C for 1 h with secondary antibody conjugated with peroxidase, and the signal was detected using chemiluminescence detection reagent. The relative protein level was calculated as the ratio of the optical density of p21WAF1 or Bcl-2 and that of β-actin.
Treated and untreated cultured HepG2 cells were harvested and centrifuged at 2 000 r/min for 5 min. The cells were resuspended in lysis buffer and centrifuged for 15 min at 15 000 g. DNA was extracted with phenol, precipitated with 100% cold ethanol, resuspended in TE buffer, electrophoresed on 1.5% agarose gel, stained with ethidium bromide, detected by ultraviolet transillumination, and photographed.
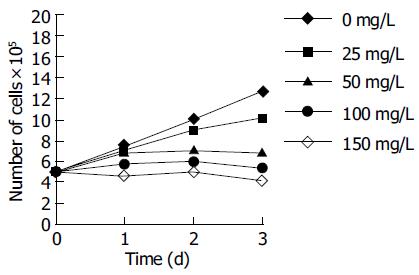
Treatment of HepG2 cells with tea pigments for 24-72 h resulted in a dose-dependent inhibition of cell proliferation. The effects of tea pigments on the proliferation of HepG2 cells are depicted in Figure 1. Tea pigments inhibited HepG2 cell growth at >25 mg/L over 3 d. The IC50, as measured by the number of viable cells 72 h after the addition of tea pigments, was 50 mg/L.
Flow cytometric analysis of HepG2 cells treated with 100 mg/L tea pigments showed a G1 arrest. The percentage of cells in the G1 phase rose from a baseline of 38.0-55.2% after 24 h of treatment and increased from 37.5% to 76.7% after 72 h (Table 1).
| Tea pigments(mg/L) | D 1 | D 2 | D 3 | ||||||
| G1 (%) | S (%) | G2/M (%) | G1 (%) | S (%) | G2/M (%) | G1 (%) | S (%) | G2/M (%) | |
| 0 | 38 | 48 | 14 | 47.1 | 39.5 | 13.4 | 37.5 | 50.3 | 12.2 |
| 100 | 55.2 | 27.8 | 17 | 68.7 | 22 | 9.3 | 76.7 | 10.3 | 13 |
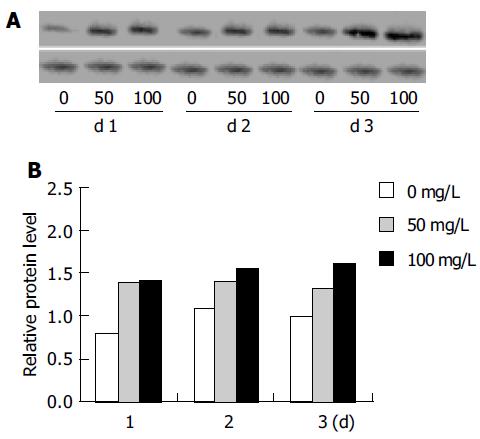
Western blot analysis of cell extracts from tea pigments-treated HepG2 cells showed upregulation of p21WAF1, an inhibitory protein that could modulate cell growth and cell cycle. The result of a typical experiment is shown in Figure 2. To obtain a quantitative value for the protein expression of p21WAF1, the optical density of p21WAF1 was measured. The ratio of p21WAF1 to β-actin was calculated, and the relative protein level of p21WAF1 was measured as presented in Figure 2, which clearly shows the upregulation of p21WAF1.
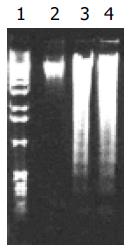
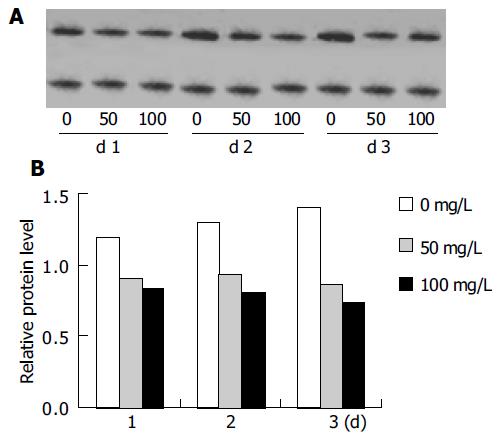
Firstly, agarose gel electrophoresis was used to demonstrate low-molecular-weight DNA ladder formation. Nuclear DNA fragmentation, a classical feature of apoptotic cell death, was clearly shown in HepG2 cells with 100 mg/L tea pigments over 3 d (Figure 3). Then, we observed the expression of Bcl-2 protein, a known inhibitor of apoptosis. Western blot analysis showed downregulation of Bcl-2 protein. The result of a typical experiment is shown in Figure 4. The ratio of Bcl-2 to β-actin was calculated. The relative protein level of Bcl-2 was measured as presented in Figure 4.
Green tea and its polyphenols have been demonstrated as chemopreventive agents in a number of experimental models[6]. However, the mechanism of chemoprevention by black tea compared with that of green tea is not quite clear. Tea pigments used in this study are the main constituents of black tea, mainly theaflavins and thearubigins. Morse et al[7] demonstrated that theaflavins inhibit NMBA-induced esophageal tumorigenesis in rats. Another study showed that black tea constituents inhibit NNK-induced lung tumorigenesis in A/J mice[8]. Previously, we also found that tea pigments had an inhibitory effect on liver precancerous lesions in rats[5]. However, the exact mechanism is unclear.
Eukaryotic cell cycle is regulated by signal transduction pathways mediated by a series of cell-cycle regulators. Cyclins are positive regulators of cell-cycle progression and function by forming a complex with and activating cyclin-dependent kinases (CDKs). CDK inhibitors are negative regulators of cell cycle and bind to and inhibit the activity of cyclin-CDK complexes[9]. p21WAF1 has gained much attention as a universal inhibitor of cyclin-dependent kinases, and overexpression of p21WAF1 has been shown to induce tumor cell growth arrest and apoptosis[10]. In this study, we have demonstrated that the inhibition of HepG2 cell growth by tea pigments is accompanied with a G1 cell cycle arrest and upregulation of p21WAF1 protein. The modulation of cell-cycle regulatory protein is thus a novel effect of tea pigments, suggesting the possible molecular mechanism by which tea pigments inhibit cell growth in HepG2 cells. Lee et al[11] showed that doxorubicin, a commonly used anticancer drug, induces G1 arrest and upregulation of p21WAF1 in HepG2 cells. Qin et al[12] also demonstrated that cisplatin induces a transient G1 arrest and upregulation of p21WAF1 expression in HepG2 cells. These results are similar to the effects of tea pigments in this study. Although we have recognized that tea pigments can modulate the expression of p21WAF1, we do not fully understand how p21WAF1 and cyclin-CDK complexes are working to elicit cell growth. Experiments are being conducted in our laboratory to elucidate the exact molecular mechanism by which tea pigments inhibit cell growth.
After treatment with tea pigments, the number of HepG2 cells decreased over time, suggesting the possibility of an increase in the rate of cell death. Agarose gel electrophoresis verified this suggestion. Nuclear DNA fragmentation, a classical feature of apoptotic cell death, was clearly shown in HepG2 cells with 100 mg/L tea pigments over 3 d. Additionally, we observed the expression of Bcl-2 protein. Modern molecular biological investigations indicate that apoptosis is regulated by many oncogenes, such as bcl-2, bax and p53[13]. Bcl-2 protein is an apoptosis-related protein and plays an important role in regulating apoptosis[14]. In our study, Western blot analysis showed that Bcl-2 protein expression was significantly downregulated in HepG2 cells after treatment with tea pigments. Bax is a homologous protein of Bcl-2, in the form of homopolymer (Bax/Bax) or isodipolymer (Bcl-2/Bax). Studies have demonstrated that the ratio of Bax and Bcl-2 protein influences the apoptotic rate of cells[15].
In conclusion, tea pigments inhibit cell growth and induce apoptosis in HepG2 cells, suggesting that tea pigments may be used as an ideal chemopreventive agent.
The authors thank Professor Qi-Kun Chen (Institute of Tea Science, Chinese Academy of Agricultural Sciences) for providing tea pigments.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Jia X, Han C. Effects of green tea on colonic aberrant crypt foci and proliferative indexes in rats. Nutr Cancer. 2001;39:239-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Kim ES, Hong WK, Khuri FR. Prevention of lung cancer. The new millennium. Chest Surg Clin N Am. 2000;10:663-90, v. [PubMed] |
| 3. | Katiyar SK, Ahmad N, Mukhtar H. Green tea and skin. Arch Dermatol. 2000;136:989-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 107] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | Leung LK, Su Y, Chen R, Zhang Z, Huang Y, Chen ZY. Theaflavins in black tea and catechins in green tea are equally effective antioxidants. J Nutr. 2001;131:2248-2251. [PubMed] |
| 5. | Gong Y, Han C, Chen J. Effect of tea polyphenols and tea pigments on the inhibition of precancerous liver lesions in rats. Nutr Cancer. 2000;38:81-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Chung FL. The prevention of lung cancer induced by a tobacco-specific carcinogen in rodents by green and black Tea. Proc Soc Exp Biol Med. 1999;220:244-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Morse MA, Kresty LA, Steele VE, Kelloff GJ, Boone CW, Balentine DA, Harbowy ME, Stoner GD. Effects of theaflavins on N-nitrosomethylbenzylamine-induced esophageal tumorigenesis. Nutr Cancer. 1997;29:7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Yang GY, Liu Z, Seril DN, Liao J, Ding W, Kim S, Bondoc F, Yang CS. Black tea constituents, theaflavins, inhibit 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK)-induced lung tumorigenesis in A/J mice. Carcinogenesis. 1997;18:2361-2365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 72] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Shah MA, Schwartz GK. Cyclin-dependent kinases as targets for cancer therapy. Cancer Chemother Biol Response Modif. 2003;21:145-170. [PubMed] |
| 10. | Mańdziuk S, Dudzisz-Sledź M, Korszeń-Pilecka I, Milanowski J, Wojcierowski J, Korobowicz E. Expression of p21 and bcl-2 proteins in paraffin-embedded preparations of non-small cell lung cancer in stage IIIA after Etoposide and Cisplatin induced chemotherapy. Ann Univ Mariae Curie Sklodowska Med. 2003;58:149-153. [PubMed] |
| 11. | Lee TK, Lau TC, Ng IO. Doxorubicin-induced apoptosis and chemosensitivity in hepatoma cell lines. Cancer Chemother Pharmacol. 2002;49:78-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 112] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Qin LF, Ng IO. Induction of apoptosis by cisplatin and its effect on cell cycle-related proteins and cell cycle changes in hepatoma cells. Cancer Lett. 2002;175:27-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 91] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Karczmarek-Borowska B, Filip A, Zdunek M, Korobowicz E, Wojcierowski J, Korszeń-Pilecka I, Furmanik F. The preliminary estimation of bcl-2, bcl-X(L) and p53 genes expression in locally advanced non-small cell lung cancer. Ann Univ Mariae Curie Sklodowska Med. 2003;58:124-131. [PubMed] |
| 14. | Farczádi E, Kaszás I, Baki M, Szende B. Changes in apoptotic and mitotic index, bcl2, and p53 expression in rectum carcinomas after short-term cytostatic treatment. Ann N Y Acad Sci. 2003;1010:780-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Nakamura H, Kumei Y, Morita S, Shimokawa H, Ohya K, Shinomiya K. Antagonism between apoptotic (Bax/Bcl-2) and anti-apoptotic (IAP) signals in human osteoblastic cells under vector-averaged gravity condition. Ann N Y Acad Sci. 2003;1010:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |