Published online Sep 7, 2005. doi: 10.3748/wjg.v11.i33.5109
Revised: February 15, 2005
Accepted: February 18, 2005
Published online: September 7, 2005
AIM: To explore the effect of six bile salts, including glycoc-holate (GC), glycochenodeoxycholate (GCDC), glycodeoxy-cholate (GDC), taurocholate (TC), taurochenodeoxycholate (TCDC), taurodeoxycholate (TDC), and two bile acids including cholic acid (CA) and deoxycholic acid (DCA) on esophageal cancer Eca109 cell line.
METHODS: Eca109 cells were exposed to six bile salts, two bile acids and the mixed bile salts at different concentrations for 24-72 h. 3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide (MTT) assay was used to detect the cell proliferation. Apoptotic morphology was observed by phase-contrast video microscopy and deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay. Sub-G1 DNA fragmentations and early apoptosis cells were assayed by flow cytometry (FCM) with propidium iodide (PI) staining and annexin V-FITC conjugated with PI staining. Apoptosis DNA ladders on agarose were observed. Activation of caspase-3 was assayed by FCM with FITC-conjugated monoclonal rabbit anti-active caspase-3 antibody and expressions of Bcl-2 and Bax proteins were examined immunocytochemically in 500 μmol/L-TC-induced apoptosis cells.
RESULTS: Five bile salts except for GC, and two bile acids and the mixed bile salts could initiate growth inhibition of Eca109 cells in a dose- and time-dependent manner. TUNEL, FCM, and DNA ladder assays all demonstrated apoptosis induced by bile salts and bile acids at 500 μmol/L, except for GC. Early apoptosis cell percentages in Eca109 cells treated with GCDC, GDC, TC, TCDC, TDC, CA at 500 μmol/L for 12 h, DCA at 500 μmol/L for 6 h, and mixed bile salts at 1 000 μmol/L for 12 h were 7.5%, 8.7%, 14.8%, 8.9%, 7.8%, 9.3%, 22.6% and 12.5%, respectively, all were significantly higher than that in control (1.9%). About 22% of the cell population treated with TC at 500 μmol/L for 24 h had detectable active caspase-3, and were higher than that in the control (1%). Immunocytochemical assay suggested that TC down-regulated Bcl-2 protein level and up-regulated Bax protein level.
CONCLUSION: GCDC, GDC, TC, TCDC, TDC, CA and DCA, except for GC, can inhibit growth and induce apoptosis of esophageal cancer Eca109 cells. Activation of caspase-3, decreased Bcl-2 protein and increased Bax protein are involved in TC-induced apoptosis of Eca109 cells.
- Citation: Zhang R, Gong J, Wang H, Wang L. Bile salts inhibit growth and induce apoptosis of human esophageal cancer cell line. World J Gastroenterol 2005; 11(33): 5109-5116
- URL: https://www.wjgnet.com/1007-9327/full/v11/i33/5109.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i33.5109
Duodeno-gastro-esophageal reflux or bile reflux is involved in the pathogenesis of reflux esophagitis, Barrett’s esophagus (BE), and esophageal adenocarcinoma. Recent studies have shown that bile acids and bile salts present frequently in the refluxate of BE patients, and influence the development and persistence of metaplasia[1-3]. Bile salts can enter mucosal cells in their non-ionized lipophilic form, cause injuries to cell membranes and tight junctions, and cell necrosis[4]. However, studies have shown that different bile salts induce cell apoptosis[5-7]. Whether different bile salts induce apoptosis of esophageal cancer cells remains unknown. Therefore, it is necessary to identify the effect of bile salts on esophageal cancer cells.
Glycine-conjugated bile acids such as glycocholic (GC) and glycochenodeoxycholic acids (GCDC) induce hepatocyte apoptosis in vitro[8], whereas taurine-conjugated bile acids such as taurocholic (TC) and taurochenodeoxycholic acids (TCDC) are well tolerated by hepatocytes[9,10]. Cell apoptosis is specifically induced by hydrophobic bile salts such as deoxycholate (DC) and dihydroxy bile salt, whereas conjugated bile salt glycodeoxycholate (GDC) and trihydroxy bile salt (TDC) and its conjugate glycocholate (GC) do not induce apoptosis in colorectal cancer cell lines[6]. Caspase cleavage and activation occur as early as 30 min after the addition of DC to cells[6]. In a recent report, direct oligomerization of the Fas receptor (CD95/Apo-1) has been suggested as the primary causative mechanism of bile salt-mediated hepatocyte apoptosis[11-13]. However, CD95 is not involved in DC-induced apoptosis, but bile salt-mediated induction of mitochondrial permeability transition has been seen in colorectal cancer cell lines[6,14].
In the present study, we observed whether six bile salts and two bile acids could inhibit cell proliferation and induce apoptosis of human esophageal cancer Eca109 cells. We characterized the kinetics of bile salt-induced apoptosis of esophageal cancer cells. The difference in effects of different bile salts and bile acids on esophageal cancer cells was demon-strated. Activation of caspase-3 and expression of Bcl-2 and Bax proteins, as the key components in mitochondrial apoptosis “d eath-signal transduction” pathway[15-18], were described in bile salt-mediated apoptotic esophageal cancer cells.
DMEM was purchased from Hyclone and trypsin was from Sigma. Fetal bovine serum (FBS) was from Hangzhou Jiangbin Biotechnology Co., Ltd. GC, GCDC, GDC, TC, TCDC, TDC, CA, and DCA were from Sigma. SP HistostainTM-Plus kits and deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) kits were purchased from Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd. Mouse mAbs to Bcl-2 and Bax were from Antibody Diagnostica Inc. Annexin V-FITC apoptosis detection kit I and FITC-conjugated monoclonal active caspase-3 antibody apoptosis kit I were from BD Biosciences.
Eca109 cell lines were obtained from the Fourth Military Medical University. Cells were cultured in DMEM plus 10% FBS. All cell lines were cultured under standard tissue culture conditions.
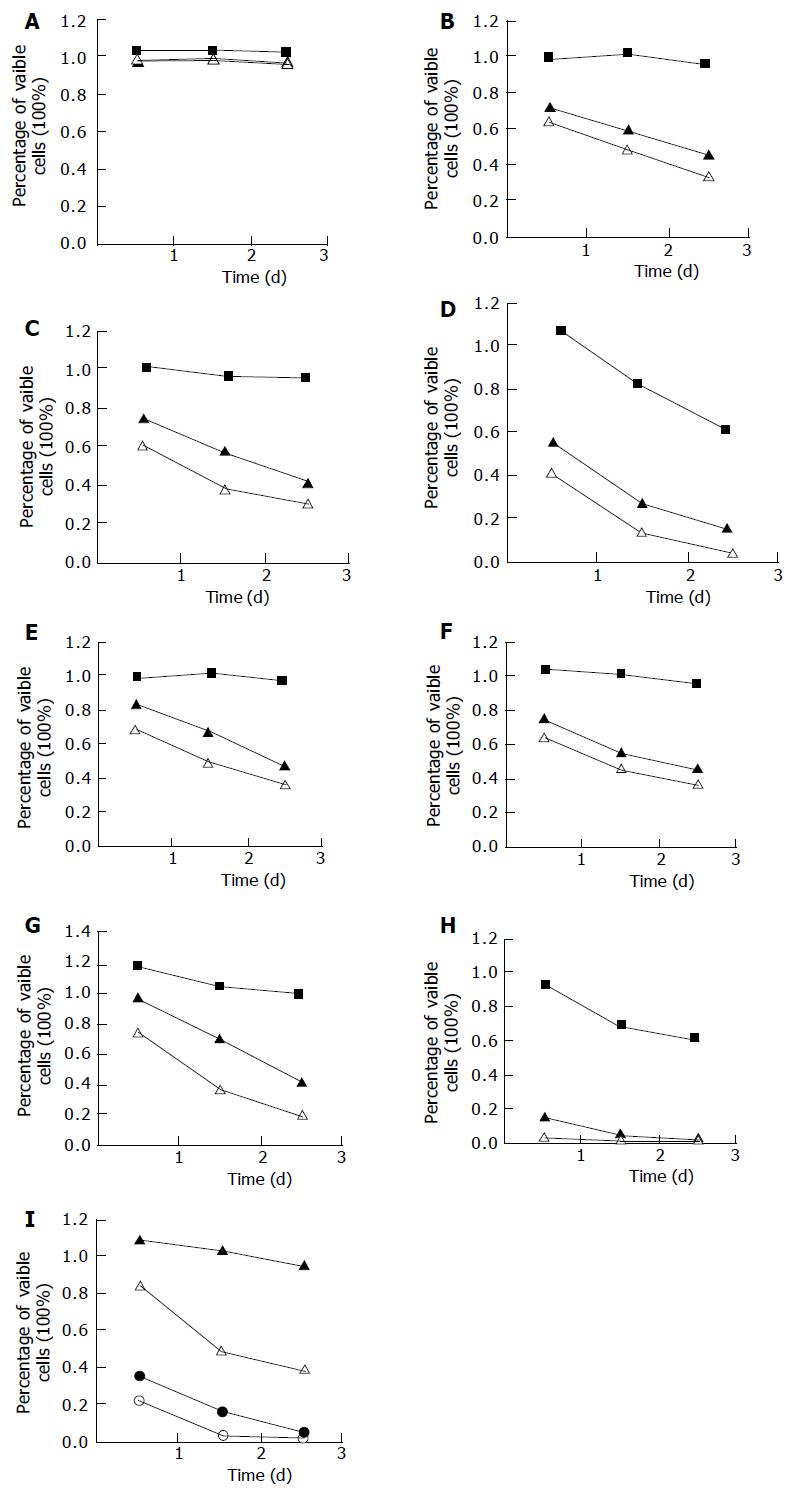
Assessment of cell proliferation MTT assay was conducted to determine the cell proliferation[19]. Eight bile salts at concentrations of 500, 250, and 50 μmol/L, and mixed bile salts at concentrations of 1 500, 1 000, 500, and 250 μmol/L, including GC, GCDC, GDC, TC, TCDC, and TDC at the ratio of 2:2:1:2:2:1, were observed in MTT assay.
Eca109 cells were seeded in a 96-well plate (4 to 6×104 cells/well). After 24 h of post seeding, cells were treated with different bile salts for 3 d, and untreated cells served as a control. Prior to the determination, 10 μL of the 2.5 g/L stock solution of MTT was added to each well. After 4 h of incubation, the culture media were discarded, and then 100 mL of DMSO was added to each well and vibrated for 10 min. The absorbance (A) was measured at 492 nm with a microplate reader. The percentage of viable cells was calculated as follows: (A of experimental group/A of control group) ×100%.
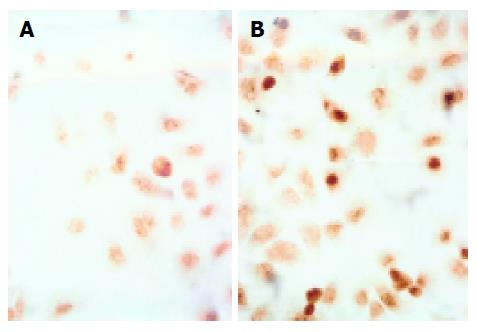
Cell apoptosis was analyzed using in situ cell death detection kit based on the terminal TUNEL technique. Cells were grown on chamber glass culture slides and treated with bile salts. To avoid disadherence of apoptotic cells, the slides were coated with poly-lysine. In brief, after the cells were treated with or without bile salts for the indicated time, they were fixed overnight in 100 g/L formaldehyde, and then treated with proteinase K and H2O2, labeled with dUTP in a humid box at 37 °C for 1 h. The cells without addition of TdT enzyme were used as negative control.
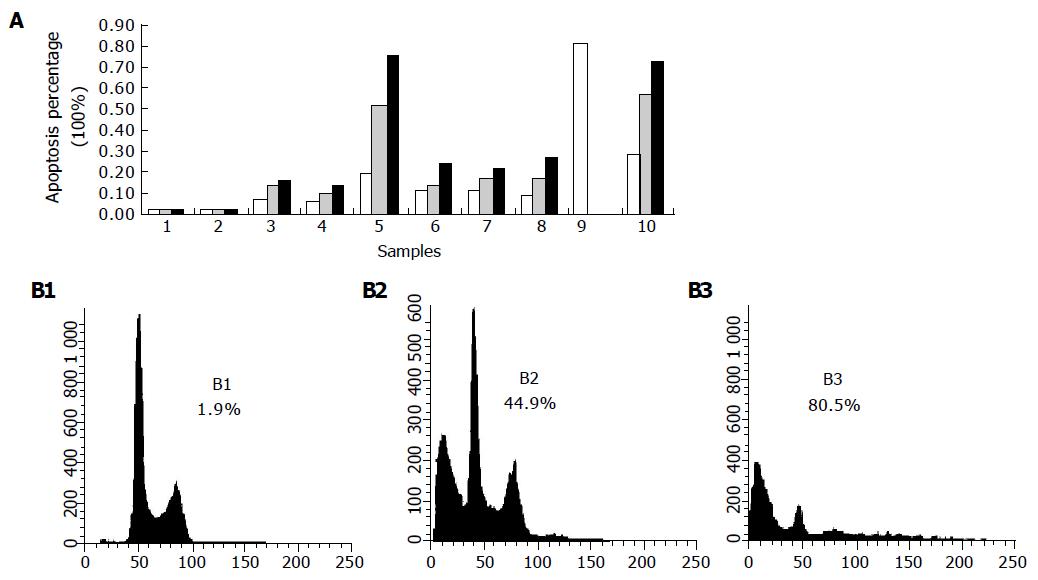
Cells from the medium supernatant and adherent cells treated with bile salts were collected and pelleted at 1 200 U/min. The harvested cells were fixed with 1 mL of 75% cold ethanol at -20 °C for a night, and then washed with PBS. Cell pellets were incubated with 10 μg/mL RNase and stained with 50 μg/mL propidium iodide (PI) for 30 min in the dark. Samples were analyzed by FACSCalibur FCM at excitation wavelength of 488 nm. In the assay, 10 000 cells were detected by FCM. The resulting histograms were analyzed by the program CELLQuest.
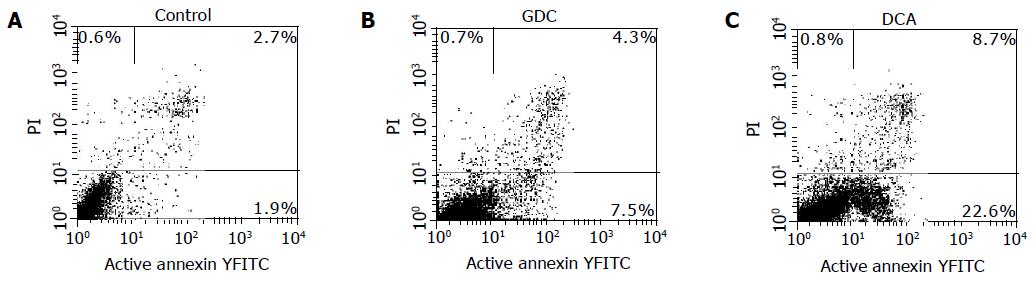
Cells from the medium supernatant and adherent cells treated with bile salts were collected and pelleted at 1 200 U/min. Pellets were washed twice with cold PBS and then resuspended in a binding buffer at a concentration of 1×106 cells/mL, and 100 μL of the solution (1×105 cells) was transferred to each of two 5-mL culture tubes. Five microliters of annexin V-FITC and 5 μL of PI were added into each 100 μL solution, the cells were gently vortexed and incubated for 15 min at RT in the dark. Four hundred microliters of 1´binding buffer was added to the sample and analyzed by FACSCalibur FCM within 1 h.
A total of 106-107 cells treated with bile salts were collected and low molecular weight DNA was extracted using cell apoptosis DNA ladder extraction kit (Beijing Dingguo Biotechnology Co., Ltd). Standard loading buffer was added, 20 μL DNA samples was run on an 1% agarose gel containing 0.1% ethidium bromide in TAE buffer (Tris 40 mmol/L, sodium acetate 20 mmol/L, and 1 mmol/L EDTA, pH 8.0).
Caspase-3 was analyzed by FCM using FITC-conjugated monoclonal active caspase-3 antibody apoptosis kit I. Cells were collected and washed with cold PBS, then resuspended in Cytofix/CytopermTM solution at a concentration of 1×106 cells/0.5 mL and incubated for 20 min on ice. Samples were washed twice with Perm/washTM buffer, and then FITC-conjugated monoclonal rabbit anti-active caspase-3 antibody was added and incubated for 30 min at room temperature. The marked samples were analyzed by FACSCalibur FCM.
Cells were grown on chamber glass culture slides and treated with TC at 500 μmol/L. To avoid disadherence of apoptotic cells, the slides were coated with poly-lysine. Slides with cells were fixed in 65% cold acetone for 20 min. Bcl-2 and Bax were stained with the mAb using standard immunocytochemical techniques. Positive-staining-area percentages were calculated using a cell image analysis system (Qwin550CW). A mean of 20 adjacent fields, at a magnification of ×400, was analyzed for each section[21].
All values were expressed as mean±SD. Statistical differences between means were calculated by Student’s t-test and χ2 test. P<0.05 was considered statistically significant.
The influence of different bile salts on the apoptosis of Eca109 cells was established. Morphological criteria of cell apoptosis, such as membrane blebbing, cell shrinkage, nuclear conden-sation and fragmentation, were assessed by phase-contrast video microscopy. Morphological changes were observed 3 h after the addition of DCA at 500 μmol/L, and 24 h after the addition of the other bile salts at 500 μmol/L, except for GC.
The experimental Eca109 cells were treated with eight kinds of bile salts and mixed bile salts at different concentrations as described above. The cell viability was determined by MTT assay (Figure 1). Except for GC, the other bile salts or bile acids inhibited the growth of esophageal cancer cells in a dose- and time-dependent manner. Cell growth was suppressed by 85% after 24 h treatment with DCA at 500 μmol/L, higher than that after treatment with other bile salts and bile acid at the same concentration (P<0.01). Cell growth was suppressed by 65% and 95% after 24- and 72-h treatment with mixed bile salts at 1 000 μmol/L, by 59% and 94% after 24- and 72-h treatment with TC at 500 μmol/L, which were higher than those after treatment with the other five kinds of bile salts.
TUNEL assay revealed that bile salts, except for GC, induced apoptosis of Eca109 cells. When treated with GC, GCDC, GDC, TC, TCDC, TDC, CA at 500 μmol/L for 24 h, DCA at 500 μmol/L for 12 h, and mixed bile salts at 100 μmol/L for 24 h, the positive-cell percentages were 2%, 11%, 9%, 25%, 11%, 12%, 15%, 43% and 30%, respectively. Compared to 2% in control, the percentages were higher in the cells treated with bile salts (P<0.05) except for GC. Figure 2 shows the image of cells treated with 500 μmol/L TC for 24 h.
Eca109 cells treated with bile salts and bile acids at 500 μmol/L and mixed bile salts at 1 000 μmol/L were stained with PI and analyzed by FCM. Except for GC, the bile salts induced cell apoptosis in a time-dependent manner (Figure 3A). When treated with DCA at 500 μmol/L, the apoptotic cell percentage reached 44.9% after 12 h and 80.5% after 24 h (Figure 3B). After 24 and 72 h, the percentages were 27.7% and 72.4% after being treated with mixed bile salts at 1 000 μmol/L, and 19.7% and 75.5% after being treated with TC at 500 μmol/L, respectively.
Early apoptotic cells were annexin V-FITC-positive and PI-negative[20]. Early apoptotic cells increased in the cells treated with bile salts except for GC. The percentages of early apoptotic cells of Eca109 cells treated with GC, GCDC, GDC, TC, TCDC, TDC, CA at 500 μmol/L for 12 h, DCA at 500 μmol/L for 6 h, and mixed bile salts at 100 μmol/L for 12 h were 1.9%, 7.5%, 8.7%, 14.8%, 8.9%, 7.8%, 9.3%, 22.6%, and 12.5%, respectively. Some of them are shown in Figure 4. Compared to 1.9% in control, the percentages were higher in the cells treated with bile salts (P<0.05) except for GC.
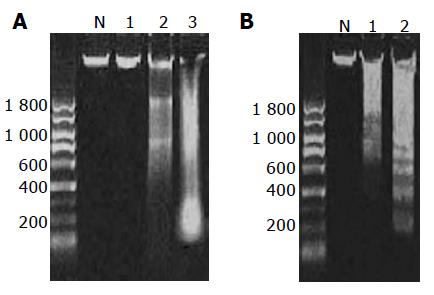
In this study, we observed DNA ladder formation in cells treated with GC, TC, and DCA at 500 μmol/L for 24 h, and mixed bile salts at 1 000 μmol/L for 24 and 48 h (Figure 5).
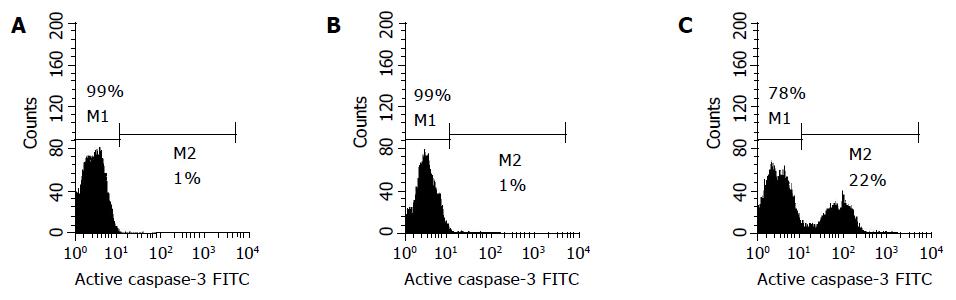
We analyzed active caspase-3 in Eca109 cells treated with TC and GC at 500 μmol/L for 24 h by FCM with FITC-conjugated monoclonal active caspase-3 antibody (Figure 6). In control and cells treated with GC at 500 μmol/L, 1% was found to be primarily active caspase-3 cells, and there was no difference between the two groups (P>0.05). But about 22% of the cells treated with TC at 500 μmol/L for 24 h had detectable active caspase-3, being higher than that in the control (P<0.01).
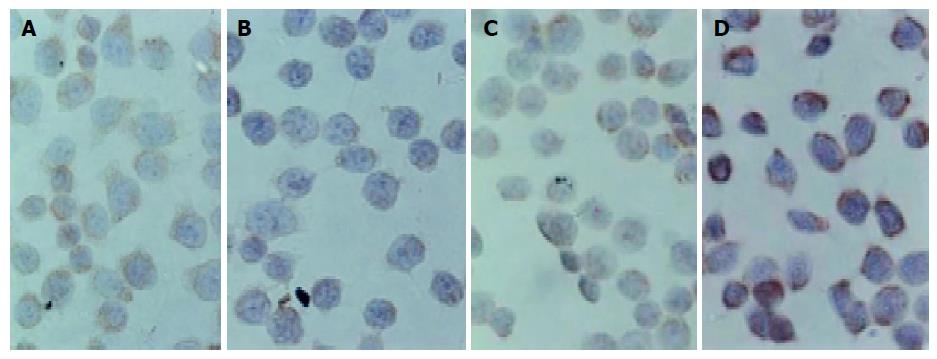
Figure 7 shows the morphologic images of cells with immunocytochemical staining of Bcl-2 and Bax proteins. Bcl-2 protein staining was weaker than Bax because the positive staining was weaker. Expression of Bcl-2 protein was reduced in 500-μmol/L-TC-treated Eca109 cells, while expression of Bax protein was increased (Table 1), suggesting that TC could down regulate Bcl-2 protein level and up-regulate Bax protein level.
Bile salts and bile acids are likely to influence the development and persistence of esophageal mucosal cell metaplasia[1-3]. It is necessary to investigate the effect of bile salts on esophageal mucosal cancer cells. In our studies, we have demonstrated that specific bile salts and bile acids at 50-500 μmol/L could inhibit growth and induce apoptosis of Eca109 cells.
Bile acids constitute 67% of normal bile, and are conjugated with glycine or taurine in normal human bile. Bile acids consisted of 40% CA, 40% CDCA, and 20% DCA. Lithocholic acid (LCA) and ursodeoxycholic acid (UDCA) is less than 5% respectively[22]. We selected GC, GCDC, GDC, TC, TCDC, and TDC, and CA and DCA in this study. The concentrations of bile salts in our study were from 25 to 500 μmol/L and those of mixed bile salts were 50 to 1 500 μmol/L, because the total bile acid concentration in esophagus of patients suffering from bile reflux is 0.89 mg/mL[23]. Six bile salts at concentration of less than 500 μmol/L are all water-soluble sodium salts in pH 7.1-7.5. Because of its hydrophobicity, the largest portion of DCA is not soluble in water, but a considerable amount of 700 μmol/L DCA is detectable in fecal water[24,25]. As this portion, at least theoretically, can get into contact with colonic epithelial cells, we selected the concentration at 25-500 μmol/L to investigate the effect of DCA on esophageal cancer cells. Our study demonstrated that less than 500 μmol/L DCA and CA could be dissolved thoroughly in the media. Based on the components of normal bile, the mixed bile salts in our study comprised GC, GCDC, GDC, TC, TCDC, and TDC at 2:2:1:2:2:1.
The present study demonstrated that five bile salts, two bile acids and the mixed bile salts except for GC, could inhibit the growth of human esophageal cancer Eca109 cells in a dose- and time-dependent manner. Our results also showed that the induced-cell death was a major reason for the cell growth inhibition. Necrosis is the major effect of bile salts on esophageal mucosal cells, by entering mucosal cells and causing injuries to cell membranes and tight junctions[4]. However, the effects of five bile salts, two bile acids and the mixed bile salts except for GC, on apoptosis-inducing activity in esophageal cancer cells were examined in our study. They caused typical apoptotic alterations including morphological changes assessed by phase-contrast video microscopy and TUNEL assay, positive annexin V staining by FCM, apoptotic sub-G1 peak by FCM with PI staining, and DNA fragmentation by gel electrophoresis.
Seven bile salts or acids and mixed bile salts have different effects between Eca109 cells and hepatocytes[8-10] and colon cancer cell lines[6]. They could induce apoptosis of esophageal cancer cells in a dose- and time-dependent manner. Conversely, hepatocyte-cytotoxic glycine-conjugated bile acids such as GC induce hepatocyte apoptosis in vitro[8], but not apoptosis of esophageal cancer cells, whereas hepatocyte-tolerated TC and TCDC[9] induce apoptosis of esophageal cancer cells. Our results also showed that TC was more toxic to esophageal cancer cells than other bile salts, suggesting that apoptosis-induced effect is correlative with the conjugated forms or structures of bile acids in esophageal cancer cells. In hepatocytes, the effect of bile salts is based on the glycine- or taurine-conjugated structure. It was reported that some hydrophobic bile acids such as TC activate PI3K-dependent survival pathways, which prevent their inherent toxicity[5]. But it seemed that PI3K was not activated by TC in esophageal cancer cells. The different mechanism of different bile salts needs to be defined by further studies.
The apoptosis “death-signal transduction” pathway is currently classified into the Fas/FasL pathway[26-28], the mitochondrial pathway[16,29], and the endoplasmic reticulum-stress activated pathway[30,31]. It was reported that direct oligomerization of the Fas receptor is the primary causative mechanism of bile salt-mediated hepatocyte apoptosis[11-13]. The signal transduction of bile salt-mediated apoptosis of esophageal cancer cells was also investigated in our study. The data demonstrate that caspase-3 is activated in TC-induced cells. Activation of caspase-3 occurs as a consequence of TC-induced apoptosis. However, caspase-3 plays a pivotal role in the three apoptosis pathways[32-34]. We further observed the expression of Bcl-2 and Bax protein. Members of the Bcl-2 family, as the key components in the mitochondrial apoptosis “death-signal transduction” pathway, are important regulators in the apoptotic pathway[15,16]. As an oncogene-derived protein, Bcl-2 confers a negative control in the pathway of cellular suicide, whereas Bax, a Bcl-2-homologous protein, promotes cell death by competing with Bcl-2[17,18]. Reduced expression of Bcl-2 protein and increased expression of Bax protein in Eca109 cells treated with TC demonstrated in this study, suggest that TC-induced apoptosis is relative with the mitochondrial death-signal pathway in Eca109 cells. As opposed to hepatocyte death mediated by bile acids, CD95 is not involved in deoxycholate-induced apoptosis of colon cancer cell lines[6]. Because the changes of CD95 were not observed in our study, our results cannot prove that Fas receptor (CD95) is not involved in TC-induced esophageal cancer cell apoptosis. Toxic bile acids may be capable of stimulating multiple pathways to initiate cell death, but further studies are needed.
In conclusion, GCDC, GDC, TC, TCDC, TDC, CA, and DCA, except for GC, inhibit growth and induce apoptosis of Eca109 cells in a dose- and time-dependent manner in vitro. Activation of caspase-3, decreased Bcl-2 protein, and increased Bax protein are involved in TC-induced apoptosis of esophageal cancer cells.
We thank Drs. Bing-Heng Zhang and Xiao-Ge Zhao in Central Laboratory for Biomedical Research of Xi’an Jiaotong University Medical School, for their help on FCM assays.
Co-first-authors: Ru Zhang and Jun Gong
Co-correspondents: Ru Zhang
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Jankowski JA, Anderson M. Review article: management of oesophageal adenocarcinoma -- control of acid, bile and inflammation in intervention strategies for Barrett's oesophagus. Aliment Pharmacol Ther. 2004;20 Suppl 5:71-80; discussion 95-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Kondo K, Kojima H, Akiyama S, Ito K, Takagi H. Pathogenesis of adenocarcinoma induced by gastrojejunostomy in Wistar rats: role of duodenogastric reflux. Carcinogenesis. 1995;16:1747-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Miwa K, Miyashita T, Hattori T. Reflux of duodenal or gastroduodenal contents induces esophageal carcinoma in rats. Nihon Rinsho. 2004;62:1433-1438. [PubMed] |
| 4. | Stein HJ, Kauer WK, Feussner H, Siewert JR. Bile acids as components of the duodenogastric refluxate: detection, relationship to bilirubin, mechanism of injury, and clinical relevance. Hepatogastroenterology. 1999;46:66-73. [PubMed] |
| 5. | Torchia EC, Stolz A, Agellon LB. Differential modulation of cellular death and survival pathways by conjugated bile acids. BMC Biochem. 2001;2:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Schlottman K, Wachs FP, Krieg RC, Kullmann F, Schölmerich J, Rogler G. Characterization of bile salt-induced apoptosis in colon cancer cell lines. Cancer Res. 2000;60:4270-4276. [PubMed] |
| 7. | Milovic V, Teller IC, Faust D, Caspary WF, Stein J. Effects of deoxycholate on human colon cancer cells: apoptosis or proliferation. Eur J Clin Invest. 2002;32:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Agellon LB, Torchia EC. Intracellular transport of bile acids. Biochim Biophys Acta. 2000;1486:198-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Torchia EC, Agellon LB. Bile acid-induced morphological changes in hepatoma cells with elevated sodium-dependent bile acid uptake capacity. Eur J Cell Biol. 1997;74:190-196. [PubMed] |
| 10. | Roberts LR, Kurosawa H, Bronk SF, Fesmier PJ, Agellon LB, Leung WY, Mao F, Gores GJ. Cathepsin B contributes to bile salt-induced apoptosis of rat hepatocytes. Gastroenterology. 1997;113:1714-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 131] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | Faubion WA, Guicciardi ME, Miyoshi H, Bronk SF, Roberts PJ, Svingen PA, Kaufmann SH, Gores GJ. Toxic bile salts induce rodent hepatocyte apoptosis via direct activation of Fas. J Clin Invest. 1999;103:137-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 411] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 12. | Sodeman T, Bronk SF, Roberts PJ, Miyoshi H, Gores GJ. Bile salts mediate hepatocyte apoptosis by increasing cell surface trafficking of Fas. Am J Physiol Gastrointest Liver Physiol. 2000;278:G992-G999. [PubMed] |
| 13. | Qiao L, Studer E, Leach K, McKinstry R, Gupta S, Decker R, Kukreja R, Valerie K, Nagarkatti P, El Deiry W. Deoxycholic acid (DCA) causes ligand-independent activation of epidermal growth factor receptor (EGFR) and FAS receptor in primary hepatocytes: inhibition of EGFR/mitogen-activated protein kinase-signaling module enhances DCA-induced apoptosis. Mol Biol Cell. 2001;12:2629-2645. [RCA] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 183] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 14. | Wachs FP, Krieg RC, Rodrigues CM, Messmann H, Kullmann F, Knüchel-Clarke R, Schölmerich J, Rogler G, Schlottmann K. Bile salt-induced apoptosis in human colon cancer cell lines involves the mitochondrial transmembrane potential but not the CD95 (Fas/Apo-1) receptor. Int J Colorectal Dis. 2005;20:103-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Tsujimoto Y, Shimizu S. Bcl-2 family: life-or-death switch. FEBS Lett. 2000;466:6-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 497] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 16. | Rupniewska Z, Bojarska-Junak A. Apoptosis: mitochondrial membrane permeabilization and the role played by Bcl-2 family proteins. Postepy Hig Med Dosw (Online). 2004;58:538-547. [PubMed] |
| 17. | Merto GR, Cella N, Hynes NE. Apoptosis is accompanied by changes in Bcl-2 and Bax expression, induced by loss of attachment, and inhibited by specific extracellular matrix proteins in mammary epithelial cells. Cell Growth Differ. 1997;8:251-260. [PubMed] |
| 18. | Lei K, Nimnual A, Zong WX, Kennedy NJ, Flavell RA, Thompson CB, Bar-Sagi D, Davis RJ. The Bax subfamily of Bcl2-related proteins is essential for apoptotic signal transduction by c-Jun NH(2)-terminal kinase. Mol Cell Biol. 2002;22:4929-4942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 400] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 19. | Saikawa Y, Kubota T, Furukawa T, Suto A, Watanabe M, Kumai K, Ishibiki K, Kitajima M. Single-cell suspension assay with an MTT end point is useful for evaluating the optimal adjuvant chemotherapy for advanced gastric cancer. Jpn J Cancer Res. 1994;85:762-765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Lecoeur H, Prévost MC, Gougeon ML. Oncosis is associated with exposure of phosphatidylserine residues on the outside layer of the plasma membrane: a reconsideration of the specificity of the annexin V/propidium iodide assay. Cytometry. 2001;44:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Coppola D, Schreiber RH, Mora L, Dalton W, Karl RC. Significance of Fas and retinoblastoma protein expression during the progression of Barrett's metaplasia to adenocarcinoma. Ann Surg Oncol. 1999;6:298-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Guoming X, Meiyun K, Jinyan L. Bile acid reflux related disease. 1st ed. Shanghai. Shanghai Sci Technol Pub. 2002;24. |
| 23. | Stein HJ, Feussner H, Kauer W, DeMeester TR, Siewert JR. Alkaline gastroesophageal reflux: assessment by ambulatory esophageal aspiration and pH monitoring. Am J Surg. 1994;167:163-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 79] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Van Munster IP, Tangerman A, Nagengast FM. Effect of resistant starch on colonic fermentation, bile acid metabolism, and mucosal proliferation. Dig Dis Sci. 1994;39:834-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 157] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 25. | Kishida T, Taguchi F, Feng L, Tatsuguchi A, Sato J, Fujimori S, Tachikawa H, Tamagawa Y, Yoshida Y, Kobayashi M. Analysis of bile acids in colon residual liquid or fecal material in patients with colorectal neoplasia and control subjects. J Gastroenterol. 1997;32:306-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Pinkoski MJ, Green DR. Fas ligand, death gene. Cell Death Differ. 1999;6:1174-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Kavurma MM, Khachigian LM. Signaling and transcriptional control of Fas ligand gene expression. Cell Death Differ. 2003;10:36-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 116] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 28. | Curtin JF, Cotter TG. Live and let die: regulatory mechanisms in Fas-mediated apoptosis. Cell Signal. 2003;15:983-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 137] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 29. | Tournier C, Hess P, Yang DD, Xu J, Turner TK, Nimnual A, Bar-Sagi D, Jones SN, Flavell RA, Davis RJ. Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science. 2000;288:870-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1351] [Cited by in RCA: 1378] [Article Influence: 55.1] [Reference Citation Analysis (0)] |
| 30. | Breckenridge DG, Germain M, Mathai JP, Nguyen M, Shore GC. Regulation of apoptosis by endoplasmic reticulum pathways. Oncogene. 2003;22:8608-8618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 556] [Cited by in RCA: 567] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 31. | Adams JM. Ways of dying: multiple pathways to apoptosis. Genes Dev. 2003;17:2481-2495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 585] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 32. | Aouad SM, Cohen LY, Sharif-Askari E, Haddad EK, Alam A, Sekaly RP. Caspase-3 is a component of Fas death-inducing signaling complex in lipid rafts and its activity is required for complete caspase-8 activation during Fas-mediated cell death. J Immunol. 2004;172:2316-2323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 33. | Yun YG, Oh H, Oh GS, Pae HO, Choi BM, Kwon JW, Kwon TO, Jang SI, Chung HT. In vitro cytotoxicity of Mokko lactone in human leukemia HL-60 cells: induction of apoptotic cell death by mitochondrial membrane potential collapse. Immunopharmacol Immunotoxicol. 2004;26:343-353. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 34. | Bustamante J, Di Libero E, Fernandez-Cobo M, Monti N, Cadenas E, Boveris A. Kinetic analysis of thapsigargin-induced thymocyte apoptosis. Free Radic Biol Med. 2004;37:1490-1498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |