Published online Aug 28, 2005. doi: 10.3748/wjg.v11.i32.5019
Revised: December 23, 2004
Accepted: December 26, 2004
Published online: August 28, 2005
AIM: To explore the expression and replication of hepatitis B virus (HBV) DNA in primary duck hepatocytes (PDHs).
METHODS: Complete HBV genome was transfected into PDHs by electroporation (transfected group, 1.19×1012 copies of linear HBV DNA/1×107 PDHs). After 1-5 d of transfection, HBsAg and HBeAg in the supernatant and lysate of PDHs were measured with the IMX System. Meanwhile, replicative intermediates of HBV DNA were analyzed by Southern blotting and Dot blotting. PDHs electroporated were used as control group.
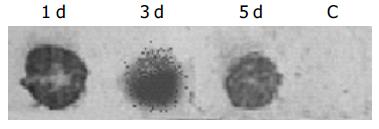
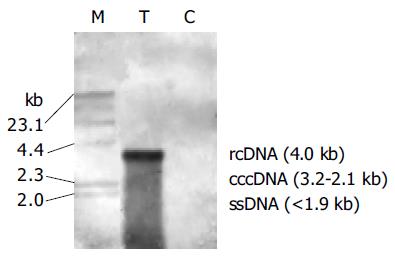
RESULTS: HBsAg in the hepatocyte lysates of transfected group was 15.24 (1 d), 14.55 (3 d) and 5.13 (5 d; P/N values, positive≥2.1) respectively. HBeAg was negative (<2.1). Both HBsAg and HBeAg were negative in the supernatant of transfected group. Dot blotting revealed that HBV DNA was strongly positive in the transfected group and negative in the control group. Southern blot analysis of intracellular total DNA indicated that there were relaxed circular (rc DNA), covalently closed circular (ccc DNA), and single-stranded (ss DNA) HBV DNA replicative intermediates in the transfected group, there was no integrated HBV DNA in the cellular genome. These parameters were negative in control group.
CONCLUSION: Expression and replication of HBV genes can occur in hepatocytes from non-mammalian species. HBV replication has no critical species-specificity, and yet hepatic-specific regulating factors in hepatocytes may be essential for viral replication.
- Citation: Yao YQ, Zhang DF, Tang N, Huang AL, Zou XY, Xiao JF, Luo Y, Zhang DZ, Wang B, Zhou WP, Ren H, Liu Q, Guo SH. Replication of hepatitis B virus in primary duck hepatocytes transfected with linear viral DNA. World J Gastroenterol 2005; 11(32): 5019-5021
- URL: https://www.wjgnet.com/1007-9327/full/v11/i32/5019.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i32.5019
Hepatitis B is one of the fatal diseases all over the world, and its pathogenesis is still unclear and its therapy appears difficult too. In recent years, reports have revealed that hepatocytes were not only the target cells infected with hepatitis B virus (HBV) and destructed by activated cytotoxic T lymphocytes, but also the immune modulating cells activated by effector cells, and even take part in the process of HBV non-cytolytic clearance[1,2]. In order to study interactions between HBV and hepatocytes, we established an in vitro model of primary culture hepatocytes from heterologous species transfected with linear HBV DNA. Since whether naked HBV DNA can replicate or express protein in non-human hepatocytes, and whether its replication process is similar to that in normal specific host are not reported, we transfected linear HBV DNA into primary duck hepatocytes (PDHs) to investigate the host-specific regulating role in the replication of HBV in hepatocytes from non-mammalian species.
Duck hepatitis B virus (DHBV) DNA and DHBsAg in duck serum were negative tested by Dot blot analysis and ELISA respectively. Hepatocytes were harvested from Chongqing ducks using an in situ collagenase perfusion technique[3]. The ducks were injected into the peritoneal cavity pentobarbital sodium (30 mg/kg body weight) and heparin (100 IU/kg body weight), the abdomen of the animals was opened and the portal vein was exposed and cannulated. Then the liver was in situ perfused at 37 °C with calcium-free, Hanks’ balanced salt solution (HBSS) for 10 min, then with 0.2 g/L collagenase type I in calcium-presenting HBSS for 15 min. The liver was removed and the cells were combed gently in tissue culture medium. Hepatocytes were centrifugated, washed, and separated from non-parenchymal cells by differential centrifugation at 50 g. Viability of hepatocytes detected by trypan blue exclusion (TBE) was about 90%, and the cells were counted.
Freshly isolated hepatocytes were transfected with linear HBV DNA, and then inoculated at a density of 3×106 cells per 25 cm2 culture flask. Culture medium was composed of RPMI 1640 with insulin (100 IU/L), penicillin, streptomycin and 100 mL/L fetal bovine serum. Cultures were incubated at 37 °C in a humidified atmosphere containing 50 mL/L CO2 and media were renewed every two days.
Plasmid pEcob 6 containing two EcoRI copies of HBV genome in a head-to-tail arrangement was used to extract the linear HBV DNA. Extraction was carried out as previously described[4]. Briefly, pEcob 6 was digested by EcoRI at 37 °C for 2 h, then fractionated by electrophoresis on 1% agarose gel. The complete HBV DNA 3.2 kb fragment was extracted and quantified for transfection.
Transfection was performed as previously described[5], electroporation conditions were optimized by Yao et al. In briefly, 4 μg linear HBV DNA was added to 1×107 hepatocytes and electroporated for about 29 ms at 220 V and 950 μF of capacitance. After gene transfer, the cells were inoculated and cultured for 5 d. The electroporated hepatocytes were used as control.
After 1-5 d of transfection, HBsAg and HBeAg in the supernatant and lysate of hepatocytes were identified using radioimmunoassay kits (Abbott Laboratories) according to the manufacturer’s instructions. The HBsAg signal/noise (P/N ratio) >2.1 was considered positive.
Intracellular total DNA was extracted 2 d after transfection. Southern blotting was used to analyze replicative intermediates of HBV DNA. Dot blotting was used to test the total HBV DNA in hepatocytes from d 1 to 5 after transfection. All procedures were performed as previously described[4].
Production of HBsAg and HBeAg was measured in culture supernatant and cell lysate was collected daily from transfected hepatocytes group. HBsAg in the lysate of transfected hepatocytes increased during the first 3 d following transfection, with HBsAg P/N value being around 15 (Table 1). HBsAg was negative in all culture supernatants of transfected hepatocytes. Both lysate and supernatant were negative for HBeAg. Both HBsAg and HBeAg in control group were negative.
| Time after transfection | 1 d | 3 d | 5 d |
| Transfected group | 15.24 | 14.55 | 5.13 |
| Control group | 1.01 | 0.93 | 1.38 |
Dot blotting revealed that total amount of HBV DNA in transfected hepatocytes was strongly positive from d 1 to 5 following transfection (Figure 1). Southern blot analysis of intracellular total DNA indicated that, there were relaxed circular (rc DNA), covalently closed circular (ccc DNA) and single-stranded (ss DNA) HBV DNA replicative intermediates in the transfected hepatocytes (Figure 2). There was no integrated viral DNA in the cellular genome.
DHBV in vivo and in vitro models have been used for the study of HBV infection and the evaluation of new anti-HBV strategies[6-10]. However, until now, there is no report on the process of viral infection, the level of DNA replication and protein expression and the mechanisms of hepatitis B virus in hepatocytes from heterologous species. Whether naked HBV DNA can replicate or express protein in nonhuman hepatocytes, and whether its replication process is similar to that in normal specific host are still unclear. We transfected linear HBV DNA into non-mammalian hepatocytes, PDH to investigate the host-specific regulating role in the replication of HBV.
Our results indicate that naked DNA of HBV can effectively replicate in PDHs and its replicative intermediates include relaxed circular DNA, covalently closed circular DNA, single-stranded DNA, and non integrated viral DNA in the cellular genome. The pattern of DNA replication of HBV is similar to that in normal permissive human cells and in liver of chimpanzee acutely infected with HBV[11], which is the same as that of DHBV replication in liver of chronically infected duck[12], and is further supported by the results of PDHs acutely or chronically infected with DHBV[3,13]. Thus complete HBV genome effectively transfected into primary hepatocytes can efficiently replicate.
Our results also show that HBV can effectively express proteins in primary duck hepatocytes, such as HBsAg, with its P/N value peak being around 15.0 1-5 d after transfection. In our study, HBsAg was found only in the lysate of duck hepatocytes, but not in the supernatant of cultured hepatocytes, which can be explained that HBsAg is insufficient to excrete in outer media of hepatocytes, and unbalance between large, mediate and small proteins of HBsAg might also affect its secretion. Furthermore, it needs further study. No HBeAg was measured in the lysate or supernatant of duck hepatocytes, which might be related to two reasons. The first, is that in duck hepatocytes there is no sufficient translation activators essential for effective activation of HBV core gene promoter. Yu and Mertz[14,15], found that HBV pre-C RNAs and pre-genome RNAs are separately regulated by two activators. The other reason is that shortly after expression, HBeAg is digested by lysozymes in duck hepatocyte plasma. The real causes need further study.
In conclusion, expression and replication of HBV genes can occur in hepatocytes from non-mammalian species, which strongly supports the idea that replication of HBV has no critical species-specificity, and hepatic-specific regulating factors in hepatocytes are essential for viral replication. Further more, the differences in inner environments of hepatocytes from different species affect and even determine the expressions of HBV genes and proteins.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Zhang DF. To pursuit novel therapeutic approaches based on the mechanism of clearance of HBV infection. Zhonghua Ganzangbing Zazhi. 2001;9:196-202. |
| 2. | Yao Y, Zhang D. Infection and replication of hepatitis B virus and its clearance mechanism: an overview. Zhonghua GanZangBing ZaZhi. 2002;10:398-400. [PubMed] |
| 3. | Yao Y, Tang N, Huang A. Study on the replication of hepatitis B virus compared with that of duck hepatitis B virus in primary duck hepatocytes. Zhonghua YiXue ZaZhi. 2001;81:1157-1161. [PubMed] |
| 4. | Yao YQ, Zhang DF, Luo Y, Zhang DZ, Huang AL, Wang B, Zhou WP, Ren H, Guo SH. An in vitro model of hepatitis B virus gene replication and expression in primary rat hepatocytes transfected with circular viral DNA. Zhonghua Gan Zang Bing Za Zhi. 2002;10:275-278. [PubMed] |
| 5. | Yao Y, Huang A, Tang N, Wang B, Zhang D. Replication and transfection of hepatitis B virus DNA into primary duck hepatocytes. Zhonghua GanZangBing ZaZhi. 2002;10:34-36. [PubMed] |
| 6. | Zhang YY, Summers J. Rapid production of neutralizing antibody leads to transient hepadnavirus infection. J Virol. 2004;78:1195-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Seignères B, Martin P, Werle B, Schorr O, Jamard C, Rimsky L, Trépo C, Zoulim F. Effects of pyrimidine and purine analog combinations in the duck hepatitis B virus infection model. Antimicrob Agents Chemother. 2003;47:1842-1852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Zoulim F, Berthillon P, Guerhier FL, Seigneres B, Germon S, Pichoud C, Cheng YC, Trepo C. Animal models for the study of HBV infection and the evaluation of new anti-HBV strategies. J Gastroenterol Hepatol. 2002;17 Suppl:S460-S463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Tang H, McLachlan A. Avian and Mammalian hepadnaviruses have distinct transcription factor requirements for viral replication. J Virol. 2002;76:7468-7472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Delmas J, Schorr O, Jamard C, Gibbs C, Trépo C, Hantz O, Zoulim F. Inhibitory effect of adefovir on viral DNA synthesis and covalently closed circular DNA formation in duck hepatitis B virus-infected hepatocytes in vivo and in vitro. Antimicrob Agents Chemother. 2002;46:425-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Guidotti LG, Rochford R, Chung J, Shapiro M, Purcell R, Chisari FV. Viral clearance without destruction of infected cells during acute HBV infection. Science. 1999;284:825-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 949] [Cited by in RCA: 914] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 12. | Ijichi K, Mitamura K, Ida S, Machida H, Shimada K. In vivo antiviral effects of mismatched double-stranded RNA on duck hepatitis B virus. J Med Virol. 1994;43:161-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Yao YQ, Zhang DF, Huang AL, Tang N, Wang B, Zhou WP, Ren H, Guo SH. Study on the replication of Hepatitis B Virus in primary hepatocytes from heterogenous species. Zhonghua Gan Zang Bing Za Zhi. 2004;12:25-28. [PubMed] |
| 14. | Yu X, Mertz JE. Promoters for synthesis of the pre-C and pregenomic mRNAs of human hepatitis B virus are genetically distinct and differentially regulated. J Virol. 1996;70:8719-8726. [PubMed] |
| 15. | Yu X, Mertz JE. Distinct modes of regulation of transcription of hepatitis B virus by the nuclear receptors HNF4alpha and COUP-TF1. J Virol. 2003;77:2489-2499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |