Published online Jul 28, 2005. doi: 10.3748/wjg.v11.i28.4414
Revised: June 23, 2004
Accepted: June 28, 2004
Published online: July 28, 2005
AIM: To study the effects of magnolol and honokiol on isolated smooth muscle of gastrointestinal tract and their relationship with Ca2+, and on the gastric emptying and the intestinal propulsive activity in mice.
METHODS: Routine experimental methods using isolated gastric fundus strips of rats and isolated ileum segments of guinea pigs were adopted to measure the smooth muscle tension. The effects of magnolol 10-3, 10-4, 10-5 mol/L, and honokiol 10-4, 10-5, 10-6 mol/L on the contractility of gastric fundus strips of rats and ileum of guinea pigs induced by acetylcholine (Ach) and 5-hydroxytryptamine (5-HT) was assessed respectively. The method using nuclein and pigment methylene blue was adopted to measure the gastric retention rate of nuclein and the intestinal propulsive ratio of a nutritional semi-solid meal for assessing the effect of magnolol and honokiol (0.5, 2, 20 mg/kg) on gastric emptying and intestinal propulsion.
RESULTS: Magnolol and honokiol significantly inhibited the contractility of isolated gastric fundus strips of rats treated with Ach or 5-HT and isolated ileum guinea pigs treated with Ach or CaCl2, and both of them behaved as non-competitive muscarinic antagonists. Magnolol and honokiol inhibited the contraction induced by Ach in Ca2+-free medium and extracellular Ca2+-dependent contraction induced by Ach. Each group of magnolol and honokiol experiments significantly decreased the residual rate of nuclein in the stomach and increased the intestinal propulsive ratio in mice.
CONCLUSION: The inhibitory effect of magnolol and honokiol on contractility of the smooth muscles of isolated gastric fundus strips of rats and isolated ileum of guinea pigs is associated with a calcium-antagonistic effect. Magnolol and honokiol can improve the gastric emptying of a semi-solid meal and intestinal propulsive activity in mice.
- Citation: Zhang WW, Li Y, Wang XQ, Tian F, Cao H, Wang MW, Sun QS. Effects of magnolol and honokiol derived from traditional Chinese herbal remedies on gastrointestinal movement. World J Gastroenterol 2005; 11(28): 4414-4418
- URL: https://www.wjgnet.com/1007-9327/full/v11/i28/4414.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i28.4414
Cortex Magnoliae officinalis is a traditional Chinese herb, belonging to "prokinetic agent" of Chinese herbs. It can improve the symptoms of abdominal distention, dyspepsia, nausea, and vomiting, etc., in gastrointestinal diseases. Magnolol and honokiol are the main components of magnoliae bark. Both can relieve spasm of smooth muscle and stop vomiting, etc. Magnolol also has an anti-allergic, anti-asthma and anti-inflammatory effect[1], and honokiol has an anxiolytic effect[2-4] and a cardiac muscle protective effect[5]. We used the isolated gastric fundus strips of rats and isolated ileum segments of guinea pigs to measure the smooth muscle tension for assessing the effects of magnolol and honokiol on the contractility of gastric fundus strips of rats induced by acetylcholine (Ach) and 5-hydroxytryptamine (5-HT), and ileum of guinea pigs induced by Ach and CaCl2. Nuclein and pigment methylene blue were used to measure the gastric retention rate of nuclein and the intestinal propulsive ratio of a nutritional semi-solid meal for assessing the effect of magnolol and honokiol on gastric emptying and intestinal propulsion.
Preparation of magnolol and honokiol: Magnolia bark (Magnolia officinalis Rehd. et Wils.) was purchased from Shenyang Medicinal Material Company. The voucher specimens of Magnolia officinalis Rehd. et Wils. was identified by Professor Sun of Shenyang Pharmacy University. Magnolol and honokiol were extracted in Shenyang Pharmacy University.
Magnolol and honokiol were dissolved in a very small amount of ethanol. In isolated and in vivo experiments the resultant solution was diluted with a 10% aqueous solution of Tween-80. Subsequently, the solution was diluted with distilled water, so that the final concentration of both ethanol and Tween-80 in the vehicle was 0.5%.
Propulsid (cisapride tablet, 5 mg/tablet) was produced by Xi'an Pharmaceutical Company Ltd (batch number: 030815020). Cisapride was crushed and dissolved in distilled water to make a 0.15 mg/mL cisapride solution.
Methylthioninium chloride injection (20 mg/2 mL) was produced by Beijing Yongkang Pharmaceutical Company Ltd.
Preparation of a semi-solid nutritious meal[6]: Ten grams of carboxymethylcellulose was added to 250 mL of distilled water. After the mixture was agitated, 16 g of milk powder, 8 g of cane sugar and 8 g of cornstarch were added to the mixture. The resulting mixture was a white semisolid paste.
Rats (200-300 g) and guinea pigs (200-300 g) of either sex were raised in cages in groups of five male mice (18-22 g) at a constant temperature (222 °C) with free access to food and water.
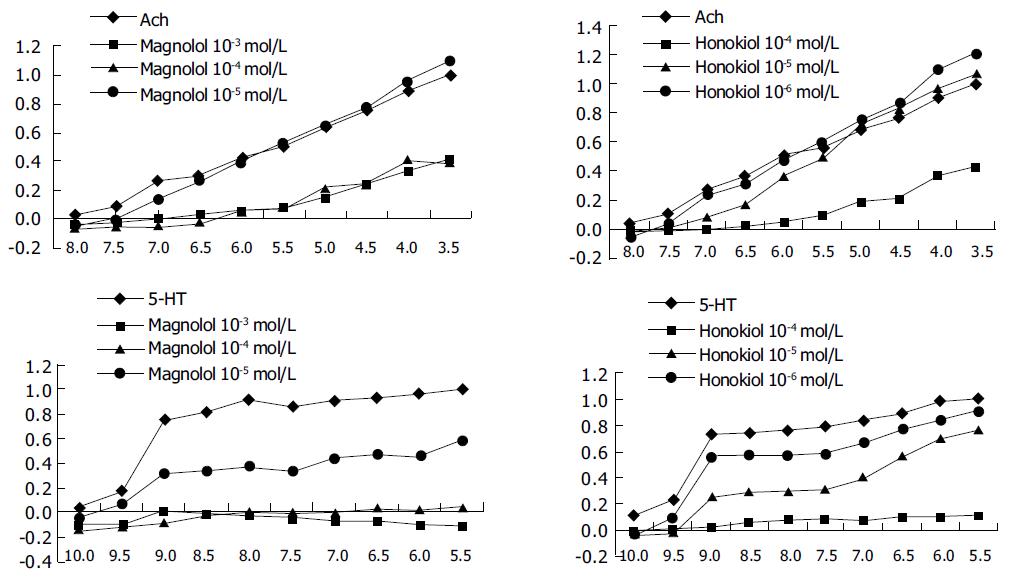
Effects of magnolol and honokiol on contractility of gastric fundus strips of rats induced by Ach and 5-HT Isolated gastric fundus strips of rats were prepared and mounted on an organ bath (Magnus bath) containing 30 mL of Kreb's solution bubbled with 95% O2+50 mL/L CO2 (pH 7.3-7.4 at 37 °C) under a resting tension of 1 g. The muscular tension, measured with a force transducer, was displayed on a MS-302 biosignal recording and analyzing system. The preparations were allowed to equilibrate for 50 min. After equilibration, 3×10-3 mol/L Ach 0.1 mL or 3×10-6 mol/L 5-HT 0.2 mL was added in the bath to activate the gastric fundus strips. When it reached its maximum contraction, it was washed with Kreb's solution to regain spontaneous contraction. Then Ach or 5-HT was added to the bath from low to high logarithmic doses to make the final respective concentrations of 10-8, 3×10-8, 10-7, 3×10-7, 10-6, 3×10-6, 10-5, 3×10-5, 10-4, 3×10-4 mol/L, or 10-10, 3×10-10, 10-9, 3×10-9, 10-8, 3×10-8, 10-7, 3×10-7, 10-6, 3×10-6 mol/L. The accumulated concentration-efficacy curve was made. When the curve reached its peak, the gastric fundus strips were washed thrice at 5-min intervals with Kreb's solution to wash the Ach out. After 15-min equilibration the drugs were added to the organ bath in respective doses (final concentrations of magnolol were 10-5, 10-4 or 10-3 mol/L, honokiol were 10-6, 10-5 or 10-4 mol/L). The accumulated concentration-efficacy curve was made after 1 min.
We made the maximum efficacy (Emax) of Ach or 5-HT as 100%, and calculated the effect percentage (E/Emax) of each concentration of Ach or 5-HT. Then the cumulative concentration-efficacy curves were drawn with E/Emax as the ordinates and the negative logarithm of final concentration of Ach or 5-HT (-log[A]) as the abscissae.
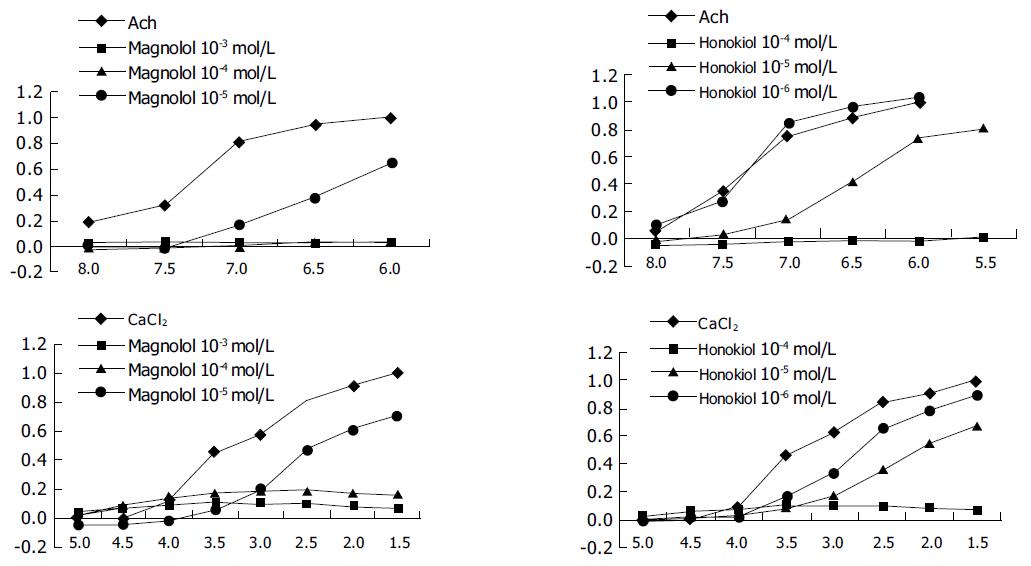
Effects of magnolol and honokiol on the contractility of the ileum of guinea pigs induced by Ach Terminal ileum segments from guinea pigs were prepared and suspended vertically in organ baths containing 30 mL of Tyrode's solution bubbled with 95% O2+50 mL/L CO2 (pH 7.3-7.4 at 37 °C) under a resting tension of 1 g. The spontaneous contractions were measured after equilibration for 50 min, 3×10-3 mol/L. Ach 0.1 mL was added in the bath to activate the ileum. When it reached its maximum contraction, it was washed in Tyrode's solution to regain spontaneous contractions. The cumulative concentration-efficacy curves of Ach were made, then the curve was remade after magnolol and honokiol were added in the organ bath respectively (the final concentrations were the same in method 1).
Effects of magnolol and honokiol on the contractility of the ileum of guinea pigs induced by CaCl2 After equilibration of guinea pig ileum segments in Ca2+-free Tyrode's solution (CaCl2 was omitted from normal Tyrode's solution) for 40 min they were changed with Ca2+-free high K+-depolarized Tyrode's solution. Ca2+-free Tyrode's solution was (the equal mole NaCl was replaced with 80 mmol/L KCl in the Ca2+-free Tyrode's solution) to make the smooth muscle depolarization after 30 min, CaCl2 was added to the bath from low to high logarithmic doses (final concentrations were 10-5, 3×10-5, 10-4, 3×10-4, 10-3, 3×10-3, 10-3, and 3×10-3 mol/L respectively) and the cumulative concentration-efficacy curves were drawn. The ileum segments were washed with the Ca2+-free Tyrode's solution till the muscular tension regained the normal contraction. After 30 min, Ca2+-free Tyrode's solution was changed with Ca2+-free high K+ Tyrode's solution and magnolol and honokiol were added respectively. After 30 min, the cumulative concentration-efficacy curves were drawn.
Effects of magnolol and honokiol on the two contractile components of Ach After equilibration for 1 h in Tyrode'ssolution, guinea pigs ileum segments were washed with Ca2+-free Tyrode's solution thrice and incubated for 30 min, then 3×10-3mol/L Ach 0.2 mL (2×10-5 mol/L) was added to the solution and it produced a brief contraction (the first or initial phasic contraction) that was induced by the release of intracellular calcium. When the ileum segments reached maximal contraction, CaCl20.2 mL (2×10-2 mol/L) was added and caused an advanced contraction (the second phasic contraction or sustained tonic component) induced by the extracellular calcium influx evoked by Ach. The ileum segments were washed with Ca2+-free Tyrode'ssolution and incubated in Ca2+-free Tyrode's solution for 30 min; meanwhile magnolol (final concentrations were 10-5, 10-4 or 10-3mol/L) or honokiol (final concentrations were 10-6, 10-5 or 10-4 mol/L) was added respectively, then Ach and CaCl2 were added successively. The guinea pig ileum segments produced the first and second contractions respectively.
Effects of magnolol and honokiol on gastrointestinal movement in mice Magnolol and honokiol were dispensed as before to the concentrations of 0.5, 2.0, and 20 mg/L. Mice were allocated randomly to a control group (administrated orally with distilled water), a cisapride group, and experimental groups. They were given orally either magnolol 0.5, 2.0, and 20 mg/L, or honokiol 0.5, 2.0, and 20 mg/L, respectively. Nuclide and pigment methylene blue were used to measure the gastric retention rate of nuclide and intestinal propulsive ratio of nutritious semi-solid meal. After having fasted for 12 h, the mice were given orally either 0.2 mL/10 g of each of the above substances or 0.2 mL/10 g of distilled water. After 30 min, each mouse was given orally a semi-solid nutritious meal containing 99mTc-DTPA 0.05 mCi/10 g and a small quantity of methylthioninium chloride. The mice were killed after 20 min, and dissected. Residual nuclide in the gastrointestinal tract was measured by a radioactivity monitor. The gastric residual nuclide rate (%) = (gastric retention nuclide/total nuclide in gastrointestinal tract)100%. Intestinal propulsive ratio (%) = (distance from sphincter of pylorus to the distal pigment methylene/distance from sphincter of pylorus to the ileocecum)100%.
Results were expressed as mean±SD. Comparisons between groups of data were made by the Student's t-test or t-test of paired comparison.
The data showed that magnolol and honokiol inhibited incompetitively the contraction of gastric fundus strips of rats induced by cumulative concentrations of Ach and 5-HT, and the effect increased with the increase of dosage. The antagonistic parameters (PD2) of magnolol and honokiol were 3.66 and 4.31 for Ach, and 5.08 and 4.91 for 5-HT respectively (Figure 1).
The data showed that magnolol and honokiol antagonized incompetitively the contraction of gastric fundus strips of rats induced by cumulative concentration of Ach, and the effect increased with the increase of dosage. The PD2 values of magnolol and honokiol were 5.09 and 4.99 respectively (Figures 2A and B).
The data showed that magnolol and honokiol antagonized incompetitively the contraction of gastric fundus strips of rats induced by cumulative concentration of CaCl2, and the effect increased with the increase of dosage. The PD2 values of magnolol and honokiol were 4.34 and 4.84 respectively (Figures 2C and D).
Magnolol and honokiol inhibited the contraction of ileal segments induced by Ach in Ca2+-free medium and the extracellular Ca2+-dependent contraction induced by Ach. Magnolol 10-5, 10-4 or 10-3 mol/L and honokiol 10-6, 10-5 or 10-4 mol/L inhibited significantly the two contractile components induced by Ach (Table 1).
In the experimental groups of magnolol 0.5, 2.0, and 20 mg/L, or honokiol 0.5, 2.0, and 20 mg/L, the gastric nuclide retention rate was significantly lower and the intestinal propulsive ratios were significantly higher than those in the control group, indicating that magnolol and honokiol could improve the gastric emptying of a semi-solid meal and intestinal propulsion in mice (Table 2).
| Group (mg/kg) | Rate of gastric residual nuclide (%) | Intestinal propulsive ratio (%) |
| Control | 50.05±12.74 | 47.92±8.49 |
| Cisapride | 22.26±9.17b | 61.92±11.77b |
| Magnolol 0.5 | 24.61±10.90b | 57.14±13.12b |
| Magnolol 2 | 26.40±12.14b | 55.46±11.26b |
| Magnolol 20 | 25.96±8.44b | 60.30±10.52b |
| Honokiol 0.5 | 24.91±11.30b | 53.57±9.54a |
| Honokiol 2 | 34.11±12.25b | 54.54±8.29b |
| Honokiol 20 | 33.22±13.64b | 53.53±9.90a |
Magnolol and honokiol are neolignan-derivatives present in Magnolia bark, which is used in the treatment of abdominal distention and vomiting. The experimental results indicated that magnolol and honokiol significantly inhibited the contractility of isolated gastric fundus strips of rats treated with Ach or 5-HT and isolated ileum of guinea pigs treated with Ach or CaCl2, and both of them behaved as non-competitive muscarinic antagonists. Magnolol and honokiol inhibited the ileal contraction induced by Ach in Ca2+-free medium and extracellular Ca2+-dependent contraction induced by Ach.
The contraction of gastrointestinal smooth muscle depends on the mediation of intracellular Ca2+, and is accomplished by the process of excitation-contraction coupling (E-C coupling). The free Ca2+ originated from the release of intracellular calcium and the restoration of extracellular calcium[7]. A high-K+ medium could depolarize the cellular membrane of ileum longitudinal smooth muscle, activate the potential-dependent calcium channel (PDC), and result in the inflow of calcium and the contraction of smooth muscles. Our experimental results indicated that magnolol and honokiol could block the transmembrane inflow of calcium through PDC, inhibit the contraction of smooth muscle, and relieve the spasm of smooth muscles.
Physical active substances Ach and 5-HT can increase the tension of gastrointestinal smooth muscle, and the agonist Ca2+ is derived from various resources. The two components of intracellular and extracellular calcium were involved in the contraction of smooth muscles induced by Ach. The first phasic contraction induced by Ach in Ca2+-free medium depended on the release of intracellular calcium. The second phasic contraction based on the first phase after the addition of calcium was caused by Ach facilitating the inflow of extracellular calcium through the receptor-operated calcium channel (ROC). In this study, magnolol and honokiol significantly inhibited the first and the second phasic contractions of smooth muscles induced by Ach, indicating that the two components of magnolia bark not only have an intracellular point of action but also inhibit the contraction of smooth muscles by blocking the inflow of calcium through ROC.
Normal gastric motility involves gastric fundus, corpus, antrum, pylorus, and antroduodenal coordination. As food is swallowed, the gastric fundus relaxes to accommodate the incoming nutrients. This is termed receptive as relaxation, which is coordinated by vagal efferent activity via nonadrenergic, noncholinergic mechanisms. As swallowing during the meal continues, the fundic filling and relaxation continue with little increase in intraluminal pressure. Gastric distention and activation of mechanoreceptor and stretch receptors stimulate vagal afferent nerve activity, which in turn modifies vagal efferent traffic.
The emptying of solid foods is accomplished by complex interplays among intra-gastric pressure, gastric peristalses, pyloroduodenal resistance, and neuroendocrine responses elicited by the specific components of the particular meal. In this study, magnolol and honokiol could significantly decrease the residual rate of nuclein in stomach and increase the intestinal propulsive ratio of semi-solid nutritious meal in mice, and there was no significant difference between them and cisapride. Combined with the study above, the functions in improving the gastric emptying and intestinal propulsive action may relate to their functions of relaxation of gastrointestinal smooth muscles.
Disorders of stomach motility and intestinal propulsion are involved in functional gastroenterological diseases such as gastroesophageal reflux disease, functional dyspepsia, irritable bowel syndrome, chronic constipation, etc.[8], gastroparesis[9], postoperative gastrointestinal atony[10], chronic intestinal pseudo-obstruction[11], and many other diseases. Prokinetic agents such as domperidone and cisapride are important therapeutic drugs[12,13]. But domperidone and cisapride have some severe side-effects, such as prolongation of the QT interval and cardiac arrhythmias[14,15]. There are rich resources of natural herbs in China, which may provide a valuable source of effective prokinetic agents.
Science Editor Wang XL Language Editor Elsevier HK
| 1. | Wang JP, Ho TF, Chang LC, Chen CC. Anti-inflammatory effect of magnolol, isolated from Magnolia officinalis, on A23187-induced pleurisy in mice. J Pharm Pharmacol. 1995;47:857-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | Kuribara H, Kishi E, Hattori N, Yuzurihara M, Maruyama Y. Application of the elevated plus-maze test in mice for evaluation of the content of honokiol in water extracts of magnolia. Phytother Res. 1999;13:593-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 3. | Kuribara H, Stavinoha WB, Maruyama Y. Behavioural pharmacological characteristics of honokiol, an anxiolytic agent present in extracts of Magnolia bark, evaluated by an elevated plus-maze test in mice. J Pharm Pharmacol. 1998;50:819-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 75] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Kuribara H, Kishi E, Hattori N, Okada M, Maruyama Y. The anxiolytic effect of two oriental herbal drugs in Japan attributed to honokiol from magnolia bark. J Pharm Pharmacol. 2000;52:1425-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Tsai SK, Huang SS, Hong CY. Myocardial protective effect of honokiol: an active component in Magnolia officinalis. Planta Med. 1996;62:503-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Francis J, Critchley D, Dourish CT, Cooper SJ. Comparisons between the effects of 5-HT and DL-fenfluramine on food intake and gastric emptying in the rat. Pharmacol Biochem Behav. 1995;50:581-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Bauer V, Holzer P, Ito Y. Role of extra- and intracellular calcium in the contractile action of agonists in the guinea-pig ileum. Naunyn Schmiedebergs Arch Pharmacol. 1991;343:58-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Hunt RH. Evolving concepts in the pathophysiology of functional gastrointestinal disorder. J Clin Gastroenterol. 2002;35:S2-S6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Tomi S, Płazińska M, Zagórowicz E, Ziółkowski B, Muszynski J. Gastric emptying disorders in diabetes mellitus. Pol Arch Med Wewn. 2002;108:879-886. [PubMed] |
| 10. | Hep A, Prásek J, Filipínský J, Navrátil P, David L, Dolina J, Dítĕ P. Cisapride (Prepulsid) in the prevention of postoperative gastrointestinal atony. Rozhl Chir. 1998;77:101-104. [PubMed] |
| 11. | Quigley EM. Chronic Intestinal Pseudo-obstruction. Curr Treat Options Gastroenterol. 1999;2:239-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Veldhuyzen van Zanten SJ, Jones MJ, Verlinden M, Talley NJ. Efficacy of cisapride and domperidone in functional (nonulcer) dyspepsia: a meta-analysis. Am J Gastroenterol. 2001;96:689-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 102] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Barone JA. Domperidone: a peripherally acting dopamine2-receptor antagonist. Ann Pharmacother. 1999;33:429-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 182] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 14. | Drolet B, Rousseau G, Daleau P, Cardinal R, Turgeon J. Domperidone should not be considered a no-risk alternative to cisapride in the treatment of gastrointestinal motility disorders. Circulation. 2000;102:1883-1885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 121] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Layton D, Key C, Shakir SA. Prolongation of the QT interval and cardiac arrhythmias associated with cisapride: limitations of the pharmacoepidemiological studies conducted and proposals for the future. Pharmacoepidemiol Drug Saf. 2003;12:31-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |